Electronic structures of impurities and point defects in semiconductors
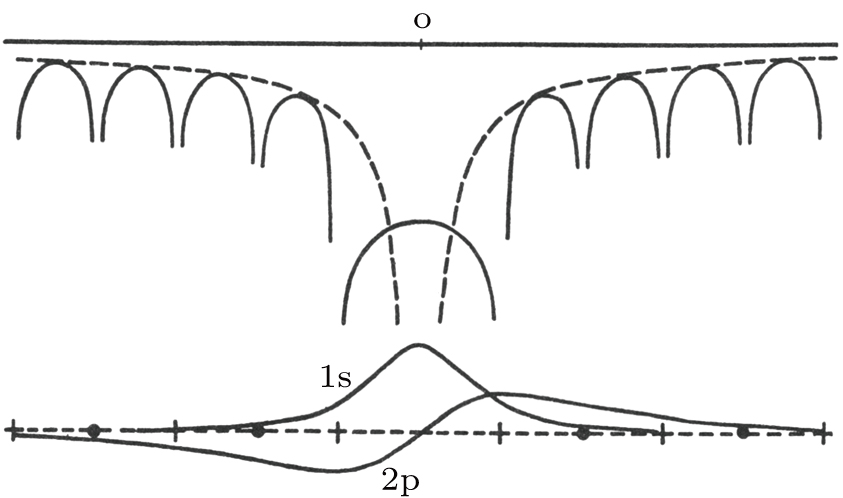
Hydrogen model for a Cl vacancy in NaCl given by Tibbs (1939).[
Electronic structures of impurities and point defects in semiconductors |
Hydrogen model for a Cl vacancy in NaCl given by Tibbs (1939).[ |
 |