† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 11474046 and 61775024), the Program for Liaoning Innovation Team in University, China (Grant No. LT2016011), the Science and Technique Foundation of Dalian, China (Grant Nos. 2017RD12 and 2015J12JH201), and the Fundamental Research Funds for the Central Universities, China (Grant No. DC201502080203).
The W18O49 nanoflowers with a diameter of 500 nm are prepared by a facile hydrothermal method. The Er–Yb: NaYF4 nanoparticles are adsorbed on the petals (the position of the strongest local electric field on W18O49 nanoflowers). With a 976 nm laser diode (LD) as an excitation source, the selectively green upconversion luminescence (UCL) is observed to be enhanced by two orders of magnitude in Er–Yb: NaYF4/W18O49 nanoflowers heterostructures. It suggests that the near infrared (NIR)-excited localized surface plasmon resonance (LSPR) of W18O49 is primarily responsible for the enhanced UCL, which could be partly reabsorbed by the W18O49, thus leading to the selective enhancement of green UCL for the Er–Yb: NaYF4. The fluorescence intensity ratio is investigated as a function of temperature based on the intense green UCL, which indicates that Er–Yb: NaYF4/W18O49 nanoflower heterostructures have good potential for developing into temperature sensors.
In the past few decades, a great deal effort has been devoted to the upconversion (UC) luminescence of rare-earth ions doped materials because of their wide applications.[1–7] The NaYF4 has been regarded as the most efficient UC matrix due to its low phonon energy and crystalline surrounding.[8–10] Although recent advances in synthesis have led to the accurate control of morphology, crystal phase, and emission colors, it is still difficult to obtain highly efficient upconversion nanoparticles. Research has been performed to improve the luminescent efficient of UC materials, such as adding different sensitizers,[11,12] changing the environment of the luminous center,[13,14] enhancing the local optical field by surface plasmon of noble metal-nanostructures,[15,16] etc. Plasmonic modulation of noble metal nanoparticles is a promising method of improving UC luminescence of nanophosphors.[2,15,16] Plasmonic nanostructures can concentrate the incoming light into a strong localized electric field distributed within a subwavelength region close to the surface of the nanostructures.[17–19] However, the widespread application has been seriously limited due to the high-cost noble metal. Recently, some heavily doped semiconductors, such as Sn-doped In2O3, WO3 − x, Cu2 − xS, and MoO3 − x, have been demonstrated to show the localized surface plasmon resonance (LSPR) phenomena.[20–24] Among them, the blue WO3 − x, a kind of nonstoichiometric tungsten oxide, exhibits an intense LSPR absorption in both the visible region and the near infrared (NIR) region.[21,25]
In this paper, we report a flower-like W18O49 prepared by the hydrothermal method. The Er–Yb: NaYF4 nanoparticles are adsorbed at the tips of petals, which are the position of the strongest local electric field. More than one order of magnitude UC enhancement is achieved through this structure. In addition, its temperature sensing property is also studied.
All chemicals were obtained from commercial suppliers and used without further purification. All reagents were of analytical grade. Rare earth chloride ReCl3 ·6H2O (Re is Y, Yb, Er, 99.9%, respectively) were purchased from Aladdin Chemistry Co. Ltd in Shanghai, China. Oleic acid (OA, C18H34O2, 90%) and octadecene (ODE, C18H36, 90%) were purchased from Alfa Aesar. Hexacarbonyl tungsten (W(CO)6) was purchased from Sigma–Aldrich. Sodium hydroxide (NaOH, ≥ 98%) and ammonium fluoride (NH4F, A.R.) were purchased from Sinopharm Chemical Reagent Co. Ltd in Shanghai, China.
In a typical process, 30 mg of W(CO)6 was dissolved into 20 mL of absolute ethanol with constant stirring to form a yellow transparent solution. Then, the solution was transferred to a 50 mL teflon-lined stainless steel autoclave, and heated up to 200 °C and kept for 10 h. The produced samples were separated from the solution by centrifugation, washed with ethanol three times, and dried in a vacuum oven.
In a typical synthesis of 20 nm nanoparticles, 1 mmol of rare earth chlorate (Y/Yb/Er = 78:20:2) with 6 mL of oleic acid and 15 mL of 1-octadecene was added into a 100 mL flask to form a mixed solution with vigorous stirring under the vacuum condition. The solution was heated to 100 °C and kept for 30 min and then cooled down to 50 °C to dissolve the rare earth. Then a methanol solution (10 mL) containing NH4F (4 mmol) and NaOH (2.5 mmol) was added dropwise, and the resulting solution was kept at 100 °C for 30 min. After methanol was evaporated, the solution was heated to 305 °C under an argon atmosphere and kept for 90 min and then cooled down to room temperature. The nanoparticles were precipitated by the addition of ethanol and isolated via centrifugation. The resulting product was washed three times with cyclohexane and ethanol (1:3) and finally dispersed into cyclohexane at a concentration of about 0.1 M.
The 3 mg of W18O49 nanoflowers and 0.1 mL of Er–Yb: NaYF4 suspended cyclohexane solution (0.1 M) were dropped into 20 mL of cyclohexane solution under ultrasonic treatment for 3 h. After that the resulting sample was separated from the solution by centrifugation, and then dried in a vacuum oven.
The dried W18O49 nanoflower samples were placed in a muffle furnace, ashing at 500 °C for 2 h to obtain WO3. The resulting sample was washed three times with ethanol and dried in a vacuum oven. The Er–Yb: NaYF4/WO3 nanoflowers were synthesized by using the same procedure mentioned above.
The phase structures of the Er–Yb: NaYF4 and Er–Yb: NaYF4/W18O49 nanoflowers heterostructures were analyzed by a SHIMADZU XRD-6000 x-ray diffractometer (XRD) with Cu K α radiation, by using the scanning mode in 2θ ranging from 10° to 80° in steps of 0.02° and at a rate of 4.0 °/min. The surface morphology of the phosphors was observed using a Hitachi S-4800 scanning electron microscope (SEM) at an acceleration voltage of 5 kV. The UV/visible/NIR absorption spectra of the samples were measured by a Lambda 750 (Perkin Elmer) combined with an integrating sphere. The UC emission properties of the products were measured by a microscope (Olympus IX71) combined with a spectrometer (PI Instrument). The excitation with a 976-nm laser passing through a laser clean-up was reflected into the objective (50 × Olympus) by a dichroic short pass filter. The UC emission was collected by the same objective and led into the spectrometer. The laser clean-up and dichroic filter were used to purify the excitation light and eliminate the laser line before the emission was detected. The optical images were achieved under 976-nm irradiation by an Olympus microscope (Scheme 1). The UC emissions from the samples at different temperatures were focused onto a Jobin Yvon iHr550 monochromator and detected with a CR131 photomultiplier tube under a 976 nm LD excitation. A home-made temperature controlling system was used to adjust the temperature of the samples from room temperature to 650 K, in which the measuring and controlling accuracy of temperature was about ± 0.5 K.
The surface morphologies of W18O49, Er–Yb: NaYF4, and Er–Yb: NaYF4/W18O49 nanoflowers heterostructures are shown in Figs.
Figure
Figure 
Figure
 | Fig. 4. (color online) Schematic energy level diagram of Er–Yb: NaYF4/W18O49 up-conversion luminescence nanoflowers particles under 976 nm LD excitation. |
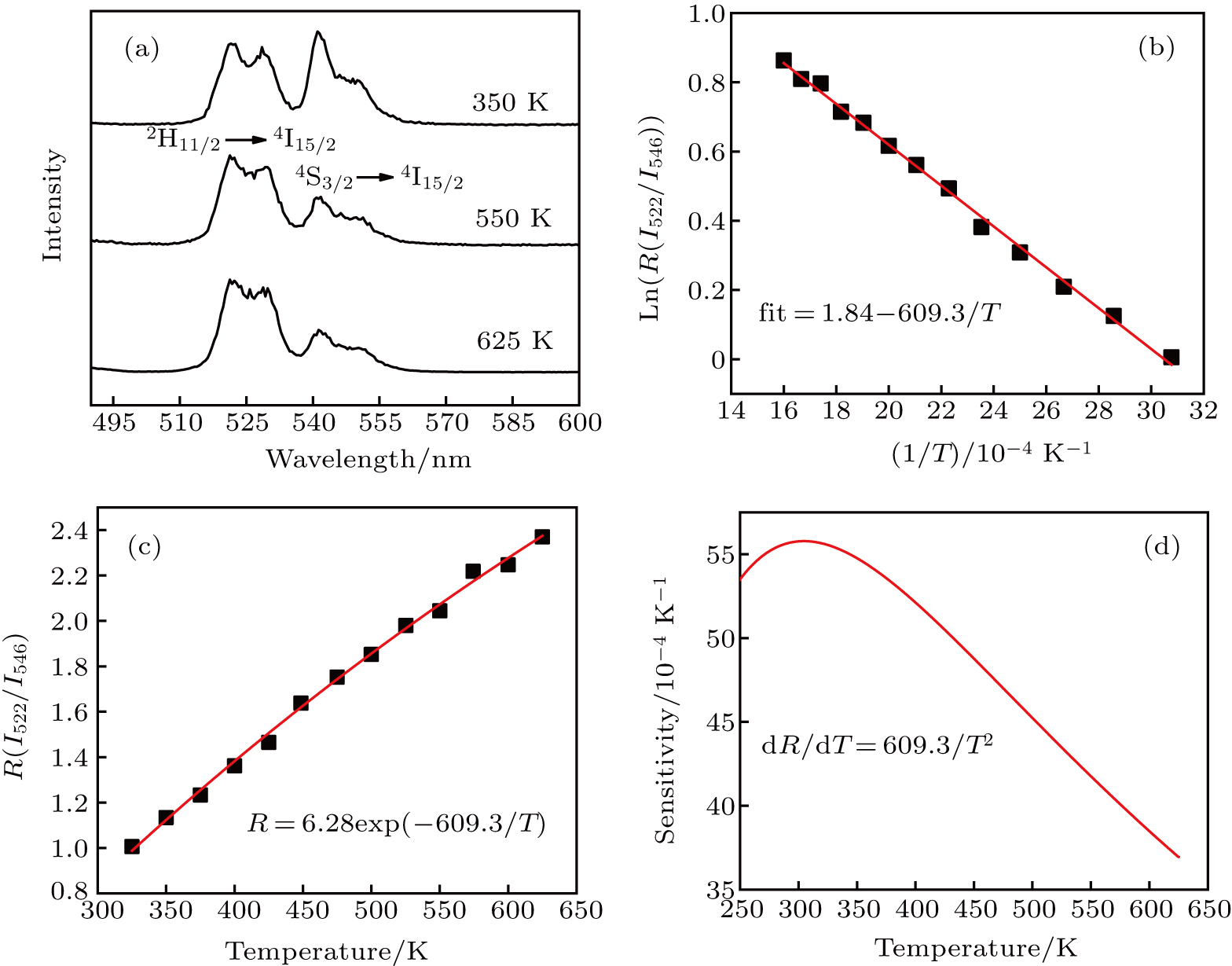
The temperature-dependent UC luminescence property is important for understanding the luminescent physical mechanism and the practical application of UC materials. Figure
 |
With regard to optical temperature-sensing applications, it is extremely important to know that the sensitivity varies with temperature. The sensor sensitivity can be defined as[23]
 |
In this research, W18O49 nanoflowers have been successfully synthesized by using a facile hydrothermal method. The nanoflowers show uniform size with the average particle diameter about 500 nm. About 40 petals are distributed evenly in all directions in one W18O49 nanoflower and the tip of the petal is about 120 nm long. The UC luminescence property of Er–Yb: NaYF4/W18O49 nanoflower heterostructure is studied under 976 nm excitation. The UC luminescence is increased by 13 fold. It suggests that the NIR-excited LSPR of W18O49 is primarily responsible for the enhanced UC luminescence, which can be partly absorbed by the W18O49, thus leading to the selective enhancement of green UC luminescence for the Er–Yb: NaYF4. The FIR of green UC is investigated as a function of temperature, demonstrating that the Er–Yb: NaYF4/W18O49 nanoflower heterostructures have potential for being developed into temperature sensors.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] |