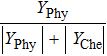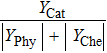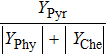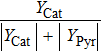Project supported by the National Natural Science Foundation of China (Grant No. 51576183) and the Fundamental Research Funds for the Central Universities, China (Grant Nos. WK2320000035 and WK2320000041).
Project supported by the National Natural Science Foundation of China (Grant No. 51576183) and the Fundamental Research Funds for the Central Universities, China (Grant Nos. WK2320000035 and WK2320000041).
† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant No. 51576183) and the Fundamental Research Funds for the Central Universities, China (Grant Nos. WK2320000035 and WK2320000041).
Phosphorus-containing compounds are the promising halon alternatives for flame inhibitions. However, some literatures suggested that the phosphorus-related inhibitors may behave as the unfavorable ones that will increase the burning velocity under lean-burn conditions, and this indeed posed potential threats to the fire prevention and fighting. To seek deeper insights into the reaction process, a numerical investigation was actualized to study the phosphorus-related effects on methane–air flames. By replacing a phosphorus-related inhibitor with the corresponding decomposed molecules, the detailed promoting and inhibiting effects of combustion were separated from the general chemical effect. A comparative study was carried out to identify the interaction between the two effects under different combustion conditions. It is observed that the promoting effect becomes the dominant factor during the reaction process when the equivalence ratio is smaller than 0.60. In this lean-burn condition, the exothermic reactions were faster than the others within the reaction chains due to the reduction of radical recombination in hydrocarbon oxidation. The results are believed to be useful for the further application and improvement of flame inhibitors.
The physical and chemical effects of halogenated fire suppressants were studied and the relative contributions of physical and chemical components on inhibitor influence were estimated.[1–4] Such researches provide the fundamental basis in selecting alternative fire suppressants to replace the halons which deplete the ozone layer,[1] and further provide the way to generate composite inhibitors composed of effective chemical inhibitors and high heat capacity diluent.[2,3] Therefore, the investigation of further insight into the physical and chemical mechanisms is of both scientific and practical magnificence.
The previous work focuses on halogenated compounds, whereas phosphorus-containing compounds (PCCs), such as dimethyl methylphosphonate (DMMP) and trimetylphosphate (TMP), are pointed out to be the effective halon alternatives which have better effects on the inhibition of hydrocarbon flame than halon,[5–7] and have been gradually used as flame inhibitors.[8,9] What the exact mechanisms of this action are has been a long standing issue. Twarowski[10–12] found that phosphine (PH3) can accelerate radical recombination in hydrogen oxidation. Korobeinichev et al. tried to explain how PCCs inhibited hydrogen flames[13] and hydrocarbon flames,[14] and MacDonald et al. conducted a few experiments and simulations to conclude that DMMP suppresses combustion by reducing the concentration of H and OH in the flames.[15] Subsequent work by Jayaweera et al. confirmed that these small molecules which reduce the concentration of free radicals in the flame are mainly PO2, HOPO2, HOPO, and PO.[16] Moreover, a series of novel phosphorus-containing flame retardants which take advantage of the chemical and physical effects have been successfully synthesized.[17–19] However, recent studies[20,21] find that adding phosphorus-containing inhibitors into the lean flames could lead to an increase in the burning velocity and have a certain effect on promoting combustion. Consequently, conducting more in-depth research on the inhibition mechanisms of PCCs is expected.
Flame suppression is caused by physical and chemical effects. The physical effects are found to be a negative factor in the combustion process due to heat capacity and dilution effects, so the unexpected combustion enhancement of PCCs originates from the chemical effect which can usually inhibit combustion since it scavenges radicals in the flame reaction chains. As a consequence, considerable controversy has been focused on how the chemical effect of phosphorus-containing inhibitors promotes the combustion, what reactions work, and whether the catalytic inhibition exists in the chemical effect. In this study, we mainly focus on these issues of PCCs.
Through experiments, it is difficult to separate the physical and chemical effects, let alone decouple the chemical effect. However, the numerical simulation method is often used to study the interaction between physical and chemical properties.[22,23] Recently, a numerical method on the chemical and physical effects of halogenated fire suppressants by setting the chemical effect on or off is utilized.[3,4] When the flame reaches a certain temperature, the inhibitors can decompose to many small molecules, and the previous method[3,4] seems to ignore the chemical effect caused by the decomposition process of the inhibitor itself. In this work, the small molecules that play major catalytic roles in flame retardation are used to replace the original phosphorus-containing inhibitor molecule. As a result, a new numerical investigation on the methane–air premixed flames adding two phosphorus-containing inhibitors (DMMP and TMP) is carried out to further decouple the chemical effect into a phosphorus-related moiety that catalyzes the flame suppression and a carbon-related moiety that initiates the decomposition and combustion. Moreover, the relative contributions of these two chemical effects on decreasing the burning velocity are quantitatively analyzed.
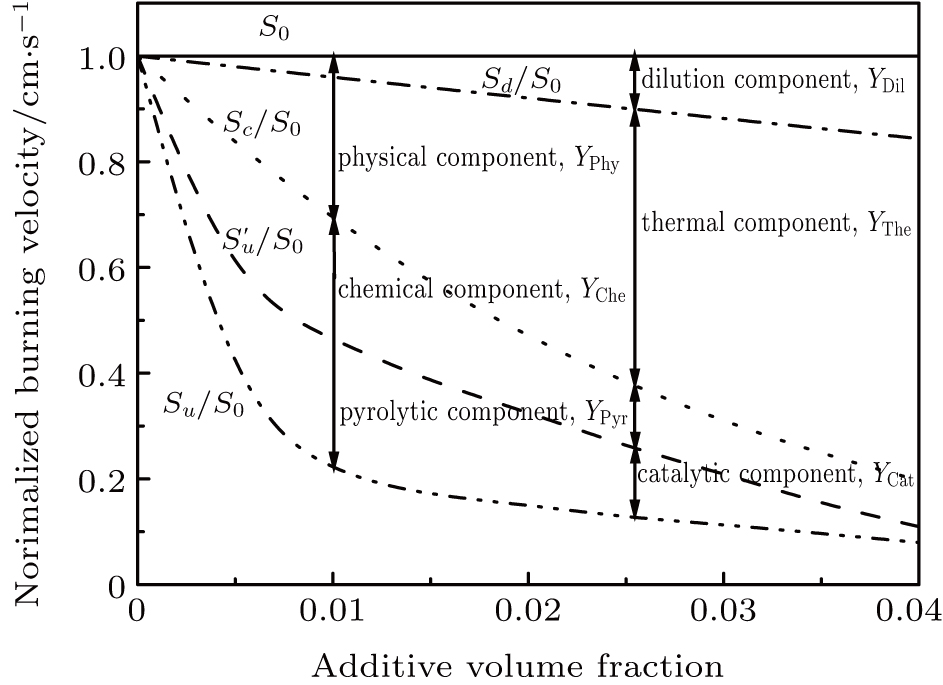
The burning velocity is one of the most basic parameters for the characterization of the flame propagation properties and also has been found to be an important parameter which characterizes the inhibition efficiency of halogen-containing flame retardants.[2] As the inhibitor concentration increases, the burning velocity decreases due to increased inhibitor influence. Babushok et al.[3] and Ren et al.[4] investigated the relationship between the physical and chemical effects of halon substitutes by using the laminar flame velocity. This method considered that the reason why the flame speed was reduced was the result of the chemical and physical effects, in which the physical effect consists of the dilution effect and thermal effect. These effects are independent, and this method can be mathematically expressed as
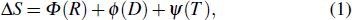 |
(i) When the flame inhibitor is not added, the laminar flame velocity of the flame is S0, and the dilution effect and the thermal effect of the flame inhibitor are both zero at this time, that is,
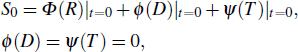 |
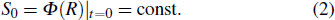 |
(ii) When the flame inhibitor is added, the laminar flame velocity of the flame is Su, and
 |
(iii) The inhibitor in the reactants is treated as an inert polyatomic molecule which does not react chemically and only the physical effect works. We can now go through the data base and turn off the chemistry of the suppressant by arbitrarily setting all rate constants that involve it in the chemical reaction mechanism and its decomposition products to be zero. The laminar flame speed is defined as Sc at this time, and
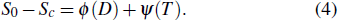 |
(iv) Compared with the inhibitor, the latent heat and specific heat capacity of N2 are very low and negligible. It is considered that N2 only has the dilution effect,[24] and N2 is used to replace the additive as flame retardant. The laminar flame speed calculated in this case is expressed as Sd, and
 |
Then the chemical effect, physical effect, thermal effect, and dilution effect of the inhibitor can be solved by Eqs. (
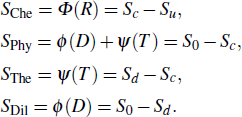 |
When the flame temperature gradually increases with the combustion, the inhibitor macromolecule decomposes into small molecules, some of which catalyze the recombination of H and OH in the flame, reducing the chain reactions and inhibiting combustion. In other words, the inhibitor is decomposed into two components of small molecules: the component of inhibiting the flame and the component of promoting combustion. Therefore, in this paper, the chemical effect of inhibitors is divided into two parts: catalytic effect and pyrolytic effect. The pyrolysis effect mentioned here includes two processes, which are the decomposition of the inhibitor into small molecules and the further reactions of such small molecules that promote combustion. Then the chemical effect of the inhibitor can be further resolved by
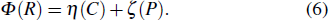 |
 |
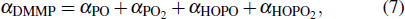 |
In addition, it was noteworthy that the total amount of PO2, HOPO2, HOPO, and PO in the combustion products accounted for more than 90% of the initial inhibitor addition in the lean flame, as shown in Table 
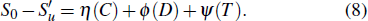 |
Therefore, in the chemical effect of the phosphorus-containing retardants, the catalytic effect and pyrolytic effect can be expressed as
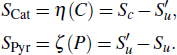 |
| Table 1. The percentage of the total quantity of PO2, HOPO2, HOPO, and PO in the products of the additive. . |
In terms of the laminar flame velocity, the effect of the flame retardant is manifested as reducing the flame speed. The flame speeds Su, Sc, Sd, and 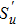
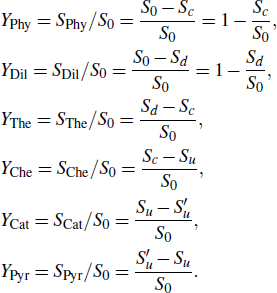 |
When the inhibition of methane–air flame is studied by DMMP and TMP, the same kinetic mechanism is used. In the calculations of the burning velocity, the GRI 3.0 mechanism[28] and Curran mechanism[29,30] were used for methane oxidation. In Fig.
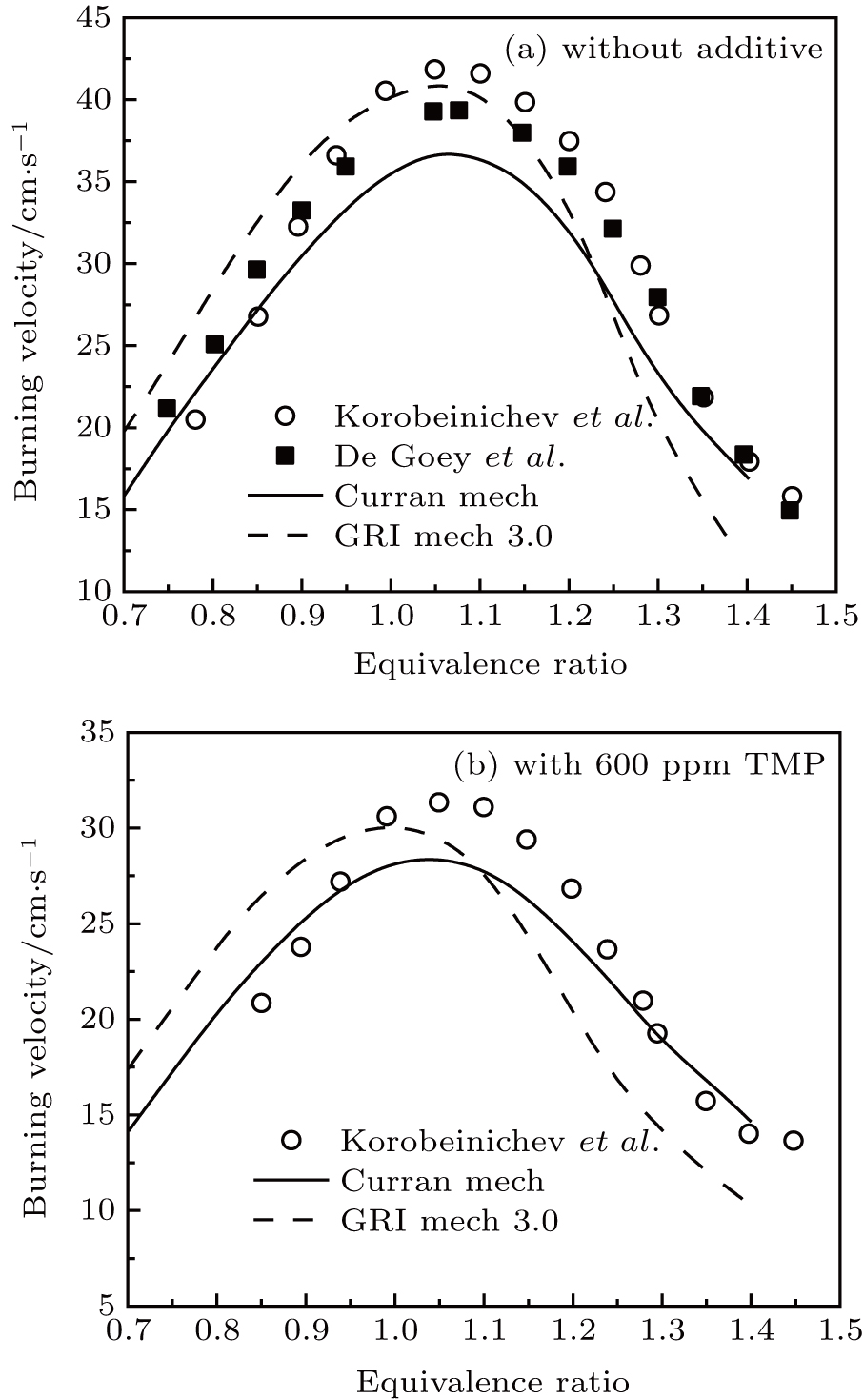 | Fig. 2. (color online) Burning velocity in CH4–air mixtures versus equivalence ratio. Symbols: experiments (circles-data from Ref. [31] and squares-data from Ref. [32]), lines: modeling (Curran mechanism[29,30] and GRI 3.0 mechanism[28]). |
The laminar flame velocity is calculated by using the PREMIX code in CHEMLIN-PRO[34] in this work. The entire calculation zone is performed in a one-dimensional region of 10 cm in length. The maximum gradient (GRAD) and maximum curvature (CURV) are set to 0.05 and 0.05, so that the final number of the generated grid is more than 500. Grid independence tests show that the absolute error of the calculated flame speed is less than 1%. Since the phenomenon of combustion promotion of phosphorus-containing inhibitors is shown in a very lean flame, the research in this work mainly focuses on the situations where the equivalence ratio ϕ is 0.40–0.65, and ϕ is selected as 0.40, 0.45, 0.50, 0.55, 0.60, 0.65, and 1.00 in initial conditions. The equivalent ratio of methane and air is calculated as
 |
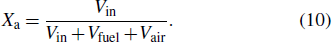 |
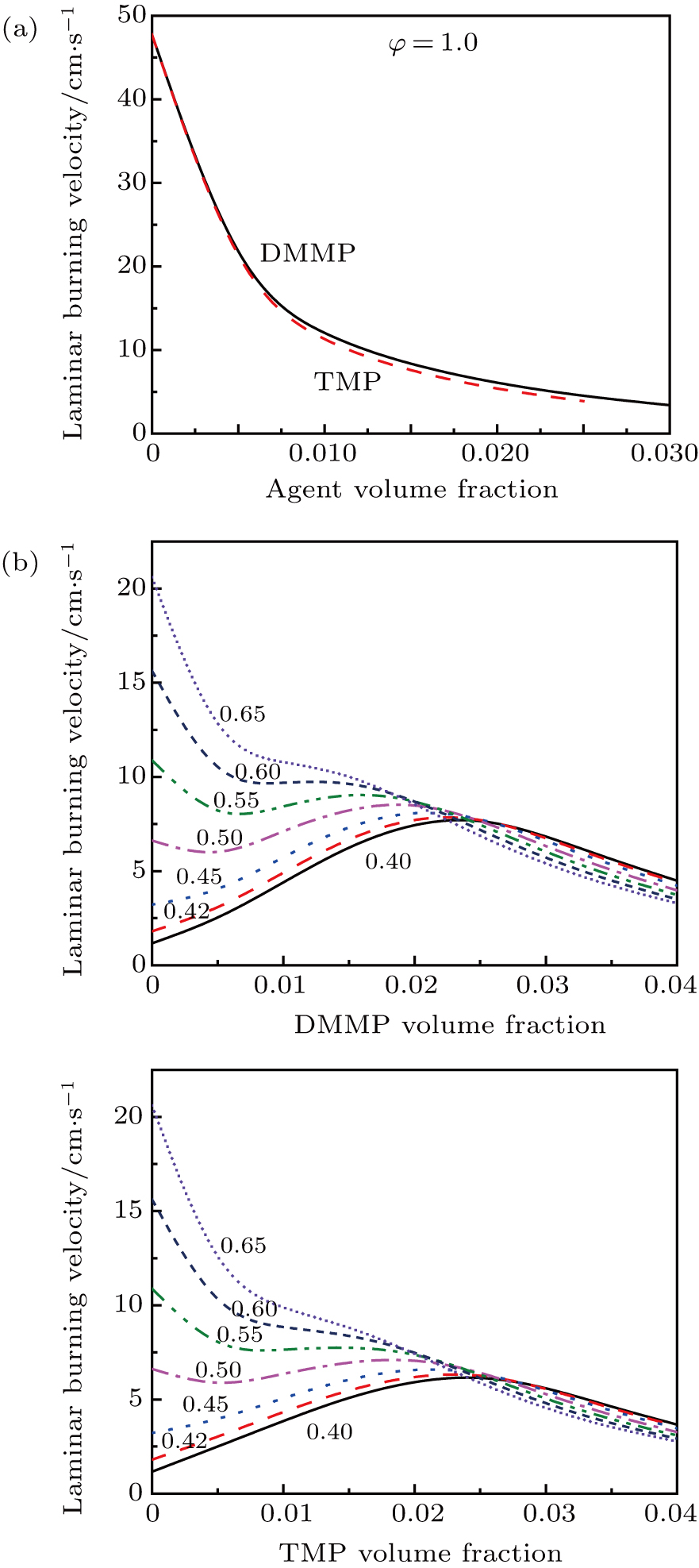
For each of the additives, DMMP and TMP, burning velocity calculations were performed under a range of equivalence ratio ϕ and an initial temperature of 373 K. Figure
In order to observe the increase of laminar burning velocity more clearly, the situation is enlarged when 0.30 ≤ ϕ < 0.50 and 0 ≤ Xa ≤ 0.02, as shown in Fig.
For the initial lean mixtures, the effect of PCCs decreases severely with ϕ decreasing, and for lean enough conditions, adding DMMP and TMP can actually increase rather than decrease the flame speed. For other flame inhibitors that also have a hydrocarbon component (for example, C3H2F3Br,[35] C2HF5,[35] and C6F12O[36]), this result has been observed and attributed to the effect of the increase in adiabatic flame temperature caused by agent-supplied fuel species addition. Figure
To demonstrate the additional fuel effect, figure
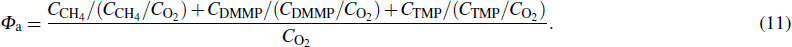 |
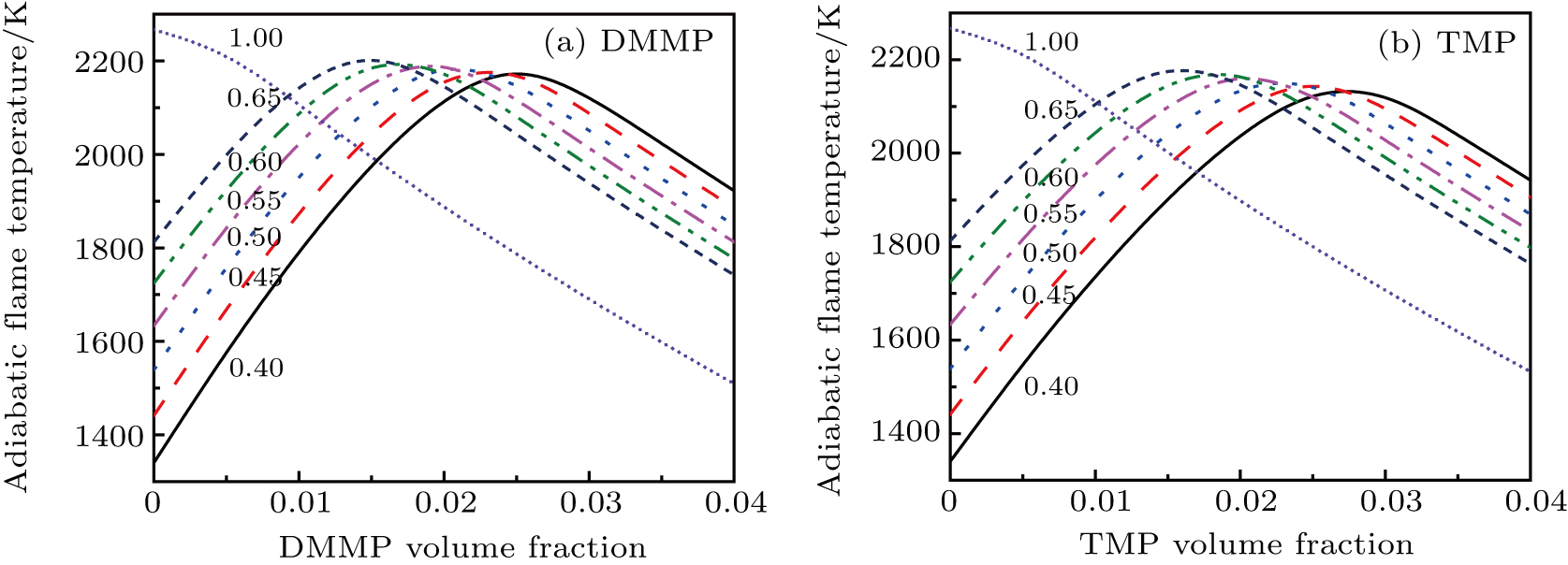 | Fig. 5. (color online) Dependence of the adiabatic combustion temperature on the inhibitor concentration at different equivalence ratios (CH4/air mixture, initial temperature 373 K). |
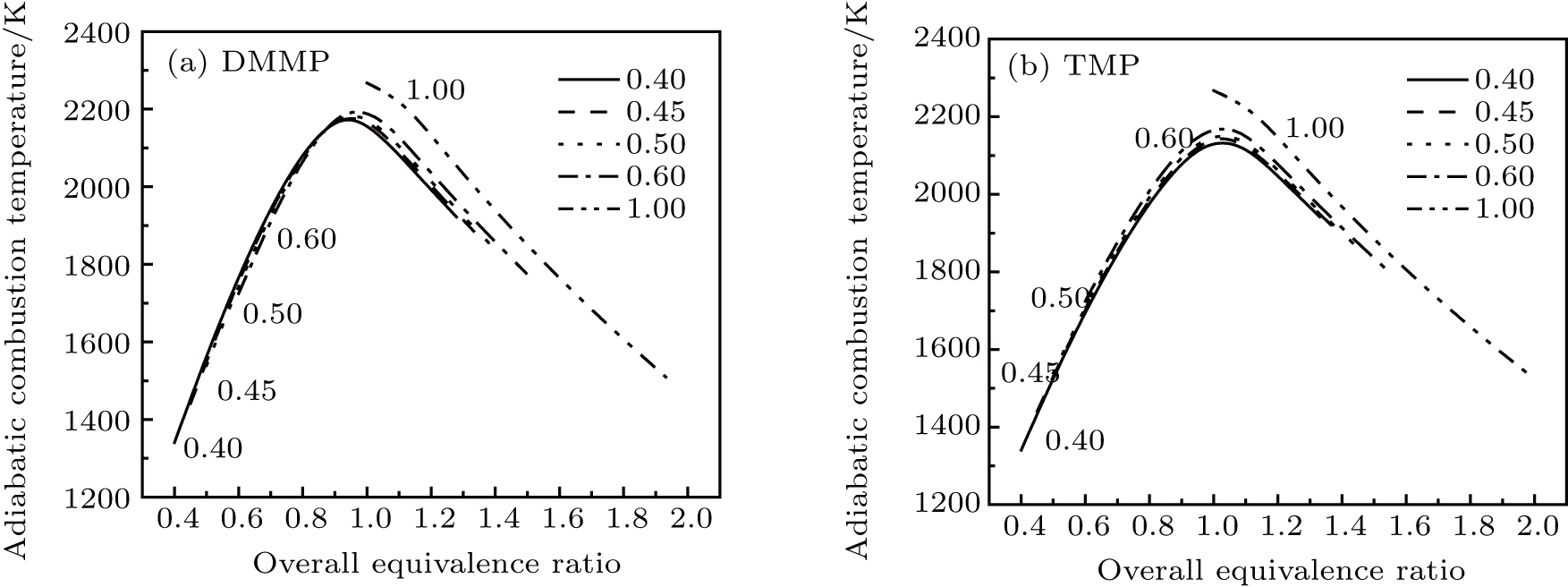 | Fig. 6. Dependence of the adiabatic combustion temperature on overall equivalence ratio for CH4–air mixture of different initial equivalence ratios. |
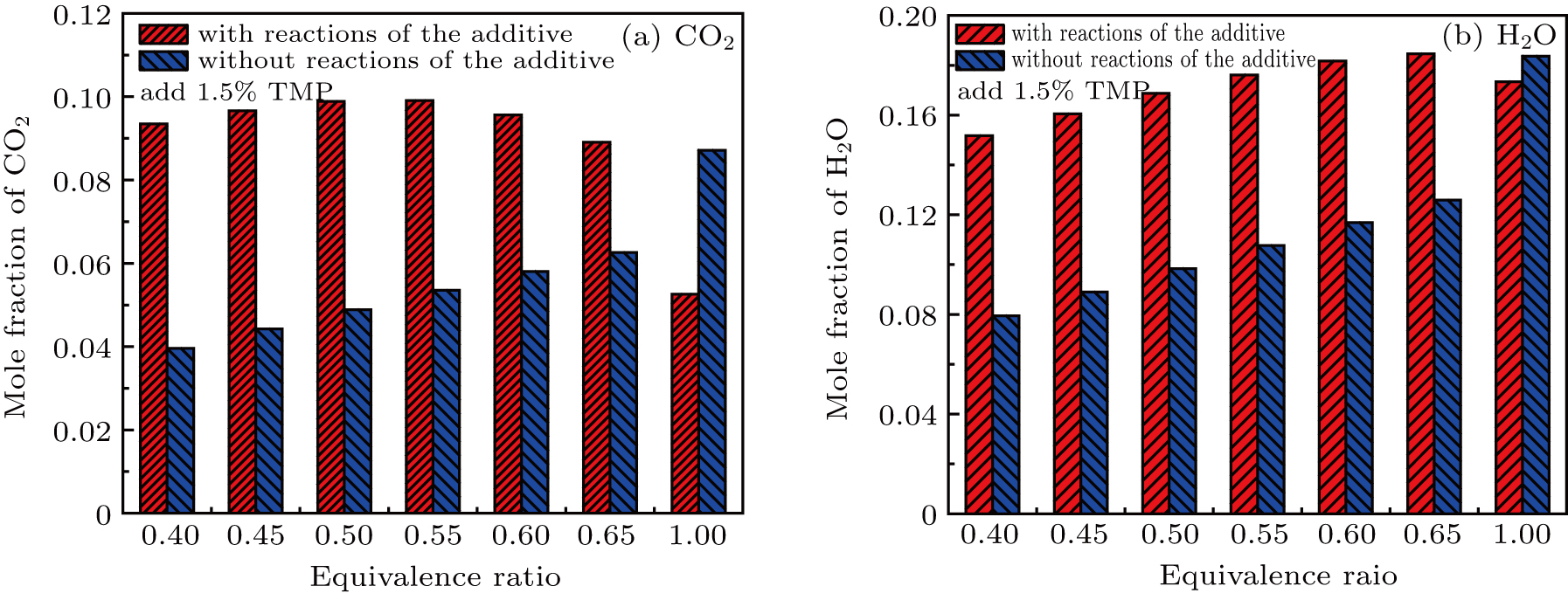
The combustion promotion of DMMP and TMP under lean conditions is fully demonstrated by the anomalous phenomenon that the flame speed and temperature increase rather than decrease. The reason for this phenomenon is that the addition of the flame inhibitor provides a new combustible material, among which the hydrocarbon component of the flame inhibitor is served as replenishment fuel for combustion. The concentrations of CO2 and H2O in the combustion products were analyzed for both cases with and without the addition of 1.5% TMP, as shown in Fig.
The effect of a flame inhibitor on a flame is composed of two parts: the physical effect, which is determined by the thermal effect and the dilution effect, and the chemical effect that is determined by the catalytic cycle reactions. Figure
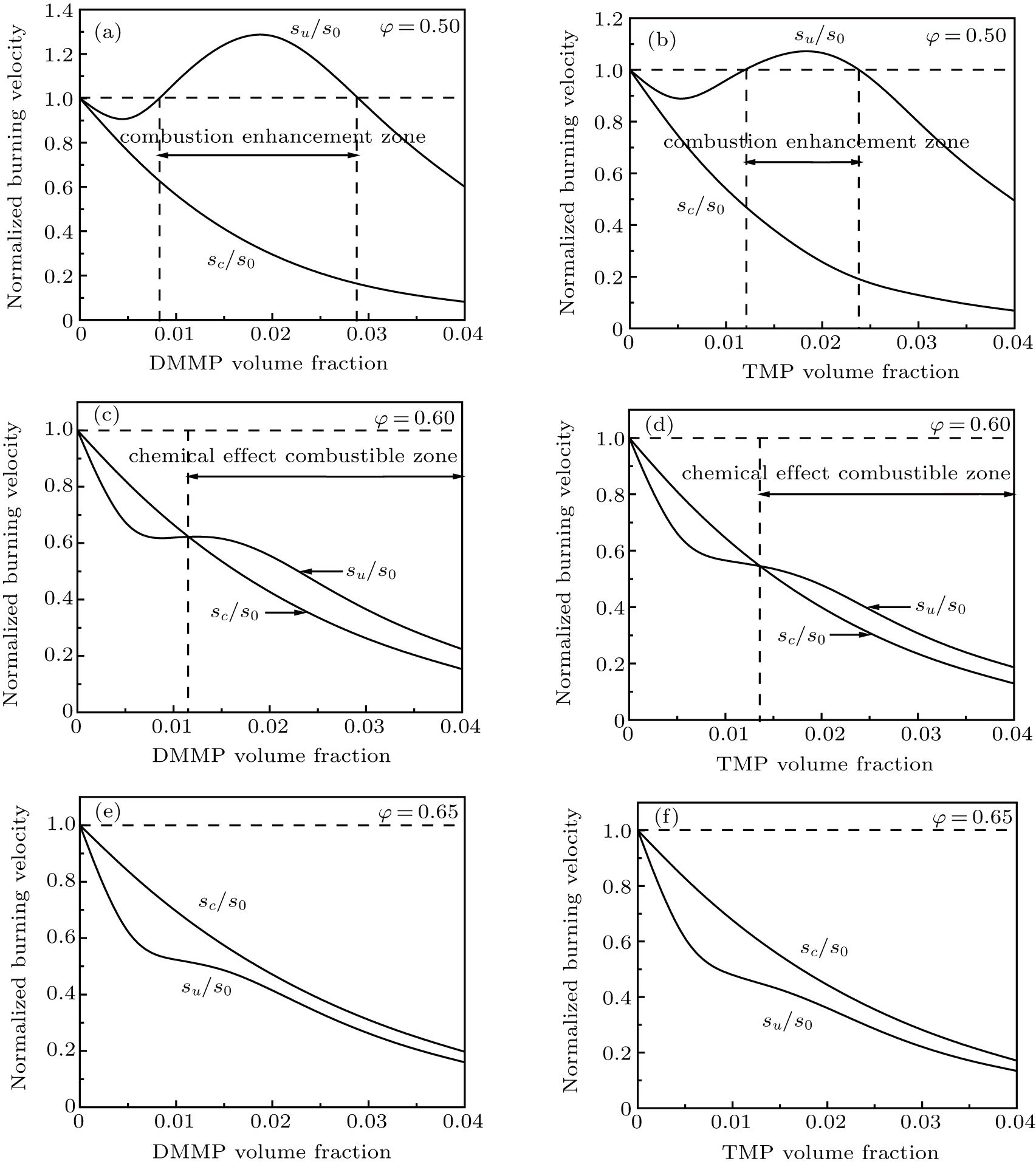 | Fig. 8. Normalized burning velocity in CH4–air mixtures with different initial equivalence ratios versus agent volume fraction. |
 | Fig. 9. (color online) The relative contributions of the physical and chemical effects for 1.5% phosphorus-containing inhibitors in CH4–air flames. |
To further explore the interrelation between the physical effect and the chemical effect, the relative contributions of them are calculated in Table
| Table 2. The relative contributions of the physical and chemical effects for 1.5% DMMP and TMP in CH4–air flames. . |
Phosphorus-containing inhibitors decompose into the phosphorus-related component and the carbon-related component. From the analysis above, it can be seen that the carbon-related component served as a supplementary fuel promotes combustion under very lean conditions, whereas the phosphorus-related component (PO, PO2, HOPO, and HOPO2) catalyzes H and OH radical recombination for flame inhibition. In this work, the effect of the thermal decomposition of PCCs and the combustion of the decomposed hydrocarbon component is called the pyrolytic effect. Therefore, the chemical effect of phosphorus-containing inhibitors is caused by the coupling of catalytic and pyrolytic effects.
By varying the rate constant, an analysis of speed sensitivity coefficients of TMP-doped CH4–air flames for the reactions involving P-containing species is shown in Fig.
Figure
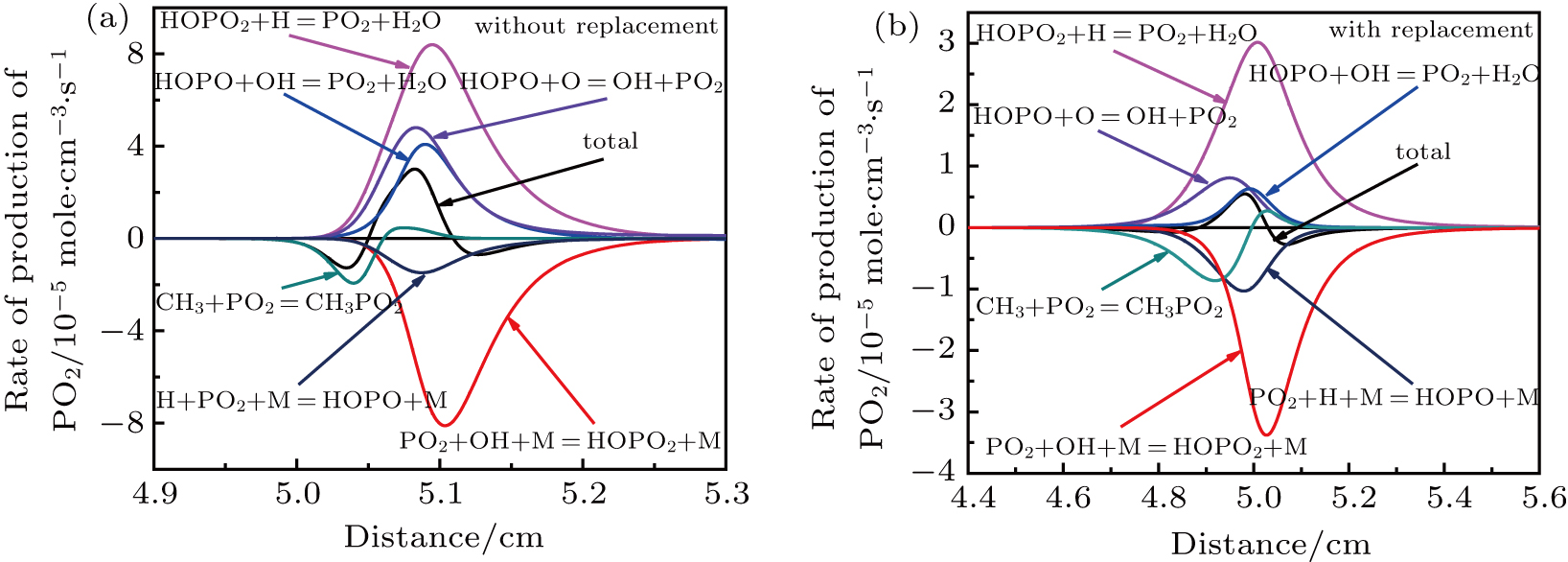 | Fig. 11. (color online) Rate of production of PO2 due to various reactions in CH4–air flame (ϕ = 0.5) doped with 1.5% TMP. |
To further evaluate the effect of the replacing model, the reaction pathways of TMP decomposition for premixed CH4–air flame (ϕ = 0.5) with 1.5% added agents are plotted in Fig.
 |
Figure
 | Fig. 13. (color online) The catalytic and pyrolytic effects of phosphorus-containing fire suppressants. |
Table
| Table 3. The relative contributions of the catalytic and pyrolytic effects for 1.5% DMMP and TMP in CH4–air flames. . |
Figure
 | Fig. 14. (color online) The physical and chemical components as a function of TMP concentration at different equivalence ratios (CH4/air mixture, initial temperature 373 K). |
Figure
PCCs exhibits a significant but undesirable promoting effect on the burning velocity in lean flames due to the chemical effect, so the physical and chemical effects are separated. To take a deeper insight into the chemical effect, this work definitely divides the initial decomposition products of phosphorus inhibitors into two components, which include the carbon-related component that supplies additional fuel to the mixture promoting combustion, and the phosphorus-related component that catalyzes radials recombination inhibiting combustion. A new approach in which the original phosphorus-containing inhibitors (DMMP and TMP) is replaced by the small P-containing molecules (PO, PO2, HOPO, and HOPO2) after decomposition turning the reactions with the carbon-related component off is proposed in the study. In this way, the chemical effect is decoupled into the pyrolytic effect and catalytic effect, and the relative contributions of these two effects to flame suppression are quantified. A numerical investigation for methane–air premixed flame with initial equivalence ratio at 0.40 ≤ ϕ ≤ 1.20 and the addition of DMMP and TMP at 0 ≤ Xa ≤ 0.04 is carried out. The results indicate that when ϕ < 0.60, the pyrolytic effect of DMMP and TMP is stronger than the catalytic effect, so that the chemical effect promotes the combustion since the flame radicals behave as the primary reaction with the hydrocarbon component of DMMP and TMP. When 0.60 ≤ ϕ < 1.00, the catalytic effect dominates the chemical effect, since the flame radicals act as the primary reaction with PO, PO2, HOPO, and HOPO2. For ϕ ≥ 1.00, the catalytic and pyrolytic effects show a synergistic effect on flame inhibition with few reactions with the hydrocarbon moiety of phosphorus-containing inhibitors.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] |