Project supported by the National Basic Research Program, China (Grant Nos. 2016YFA0202300 and 2016YFA0202302), the National Natural Science Foundation of China (Grant Nos. 61527817, 61335006, and 61378073), and the Beijing Municipal Science and Technology Committee, China (Grant No. Z151100003315006).
Project supported by the National Basic Research Program, China (Grant Nos. 2016YFA0202300 and 2016YFA0202302), the National Natural Science Foundation of China (Grant Nos. 61527817, 61335006, and 61378073), and the Beijing Municipal Science and Technology Committee, China (Grant No. Z151100003315006).
† Corresponding author. E-mail:
Project supported by the National Basic Research Program, China (Grant Nos. 2016YFA0202300 and 2016YFA0202302), the National Natural Science Foundation of China (Grant Nos. 61527817, 61335006, and 61378073), and the Beijing Municipal Science and Technology Committee, China (Grant No. Z151100003315006).
Producing hydrogen through a hydrogen evolution reaction (HER) by splitting water at the suitable overpotential is a great alternative to solving the problems of environmental pollution and the energy crisis. Molybdenum sulfide (MoS2) has attracted extensive attention as one of the most promising catalytic materials for HER. In this work, we design a facile method to in situ grow gold nanoparticles (AuNPs) on MoS2. Different numbers of AuNPs with MoS2are used to find the best catalytic activity. Due to the larger active surface area and higher conductivity of the Au–MoS2 composites, all the Au–MoS2 composites exhibit more enhanced HER electroactivity than pure MoS2. In brief, the new material architecture exhibits optimized HER activity with a low onset overpotential of 0.12 V, low Tafel slope of 0.163 V·dec−1, and an excellent stability in acidic solution.
In recent years, the serious rapidly increasing energy crisis and the associated environmental pollution have promoted the development of renewable and clean alternative energy sources.[1–3] Due to the high energy density and zero carbon emission, hydrogen has attracted attention for replacing traditional fossil fuels and been considered as a promising next-generation clean fuel resource.[4–7] Electrochemically splitting water via hydrogen evolution reaction (HER) is an attractive way to produce hydrogen and regarded as an excellent energy carrier in the future.[8–10] To date, platinum (Pt) or its alloys is considered to be one of the most effective electrocatalysts for hydrogen evolution reaction,[11–13] however their high price and extreme shortage seriously hinder their broad-scale industrial applications.[14–16] Replacing Pt with highly effective catalysts based on earth-abundant elements is a challenging research problem.[17]
Due to a similar Gibbs free energy for hydrogen adsorption of platinum,[18–20] low cost and high abundance, molybdenum disulfide (MoS2)-based electrocatalysts have attracted increasing attention.[21–25] Much work has been performed with the aim of improving the exposure of active sites of MoS2 and facilitating the electron transportation in the HER process.[26–29] For example, by using density functional theory (DFT), Hinnemann et al. demonstrated that the basal plane of MoS2 is catalytically inactive, however the edge sites are catalytically active for hydrogen evolution reaction.[30] Jaramillo et al. proved that the hydrogen evolution reaction activity of MoS2 is proportional to the length of edge rather than the coverage area.[31] Smith et al. synthesized MoSx grown on crumpled-graphene–modified carbon cloth composite catalyst, and significantly enhanced the hydrogen evolution reaction activity.[32] Huang et al. demonstrated that Pt–MoS2 hybrid nanomaterials exhibit much higher electrocatalytic activity towards the hydrogen evolution reaction than the commercial Pt catalysts with the same Pt loading.[33]
In this work, we synthesize an Au–MoS2 composite through in situ growth method. Gold nanoparticles (AuNPs) have various unique properties such as excellent conducting capability, high specific surface area and superior catalytic property.[34–37] The involvement of AuNPs can not only enhance the electronic property of MoS2, but also increase the specific surface area of the composite. To improve the electrocatalytic performances of the Au–MoS2 composites, different numbers of AuNPs with MoS2 are evaluated by using a three-electrode system. All the composites exhibit significant enhancement in catalytic activity compared with pure MoS2. To the best of our knowledge, this is the first report on synthesizing AuNPs for the MoS2 and utilizing the composite for catalyzing hydrogen evolution reaction.
Sulfuric acid (H2SO4), chloroauric acid (HAuCl4·4H2O), sodium citrate, L-cysteine, sodium molybdate (Na2MoO4), and other reagents were purchased from Alfa Aesar. Deionized water was used for preparing all solutions.
The morphologies of catalysts were characterized using field emission scanning electron microscopy (SEM) with an accelerating voltage of 15 kV (Hitachi S-4800, Japan). Transmission electron microscopy (TEM) images were achieved by a JEM-1400 operating at 120 kV. The x-ray photoelectron spectroscopy (XPS) measurement was carried on PHI Quantera (PHI, Japan), and the binding energy is calibrated with C 1s = 284.8 eV.
All electrochemical measurements were performed on a glass carbon electrode (GCE)by using a three-electrode CHI660D electrochemical workstation (Chenhua Instrument, Shanghai, China) in 0.5-M H2SO4 solution, platinum wire was used as a counter electrode, and a Ag/AgCl electrode served as a reference electrode. Typically, 6-μL AuNPs, MoS2, and Au–MoS2 composite inks were drop-casted onto the conventionally pretreated 3-mm-diameter GCEas working electrodes. In this study, all the potentials measured were verified by the reversible hydrogen electrode (RHE) according to the equation: ERHE = EAg/AgCl + 0.197 + 0.059 pH. Linear sweep voltammetry (LSV) were swept from −0.8 V to 0.4 V versus RHE was swept with a scan rate of 0.05 V/s. The current density was calculated based on the geometric area of the GCE to be 7.07 × 10−2 cm2.
In a typical synthesis of the MoS2, 0.2-g Na2MoO4 and 0.4-g L-cysteine were dissolved in 40-mL deionized (DI) water. The mixture was sonicated for 15 min. The homogeneous solution was then transferred into a 100-mL Teflon-lined stainless steel autoclave reactor, and hydrothermally treated in an oven at 180 °C for 12 h. The autoclave was then removed from the oven and naturally cooled to room temperature. The black product formed in the solution was then collected by centrifugation, washed with DI water and ethanol repeatedly many times, and then dried by lyophilization. Finally, the dried black product was dispersed in DI water with a concentration of 8 mg/mL.
Au nanoparticles were synthesized according to our previously reported method.[38] Briefly, 500-μL HAuCl4 (25.4 mM) was added into 40-mL boiling distilled water under vigorous stirring, and 900-μL trisodium citrate (0.1 M) was dropwise added into the boiling solution under constant stirring for 20 min, the color changed to wine red when the reaction was completed. Finally, the suspension was cooled down to room temperature under continuous stirring and stored at 4 °C.
Au nanoparticles (NPs) were in situ synthesised on the MoS2 nanoflower. Briefly, 0.75-mL MoS2 aqueous solution was added into 20-mL DI water followed by the addition of HAuCl4 (25.4 mM). HAuCl4 solution volumes were set to be 10, 25, 50, 100, and 150 μL. The mixture was sonicated for 15 min. Then, the solution was heated to boiling under vigorous stirring, and excess trisodium citrate (0.1 M) was dropwise added into the mixture under constant stirring for another 20 min. After the suspension was cooled down to room temperature, the products were centrifugally washed with DI water four times. Finally, the black products were dispersed in 20-mL DI water. The composites were denoted as Au–MoS2-10, Au–MoS2-25, Au–MoS2-50, Au–MoS2-100, and Au–MoS2-150, respectively.
Figure
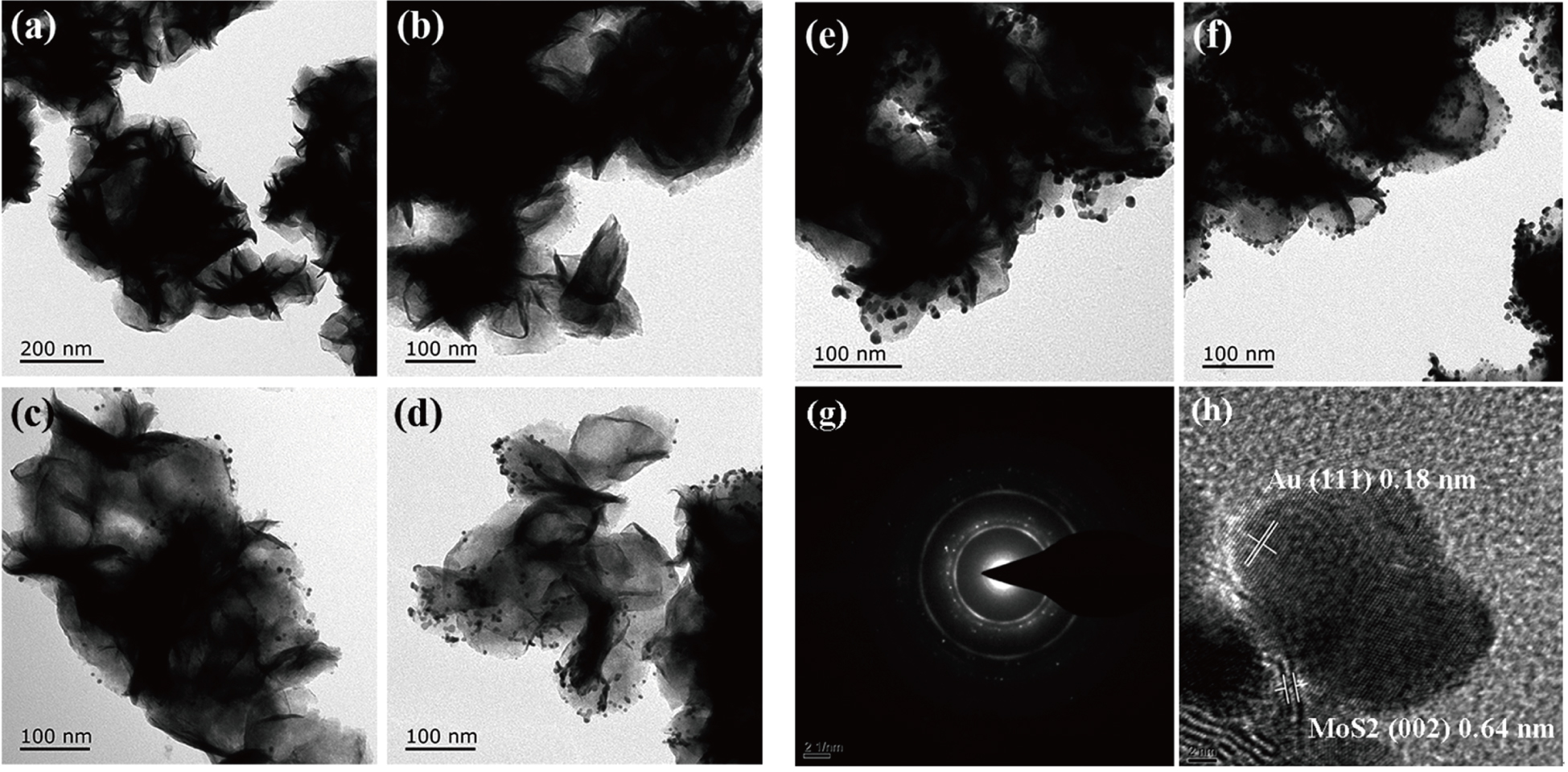 | Fig. 1. TEM images of (a) pure MoS2, (b) Au–MoS2-10, (c) Au–MoS2-25, (d) Au–MoS2-50, (e) Au–MoS2-100, and (f) Au–MoS2-150. (g) SAED patterns of Au–MoS2-100. (h) High-resolution TEM of AuNPs/MosS2. |
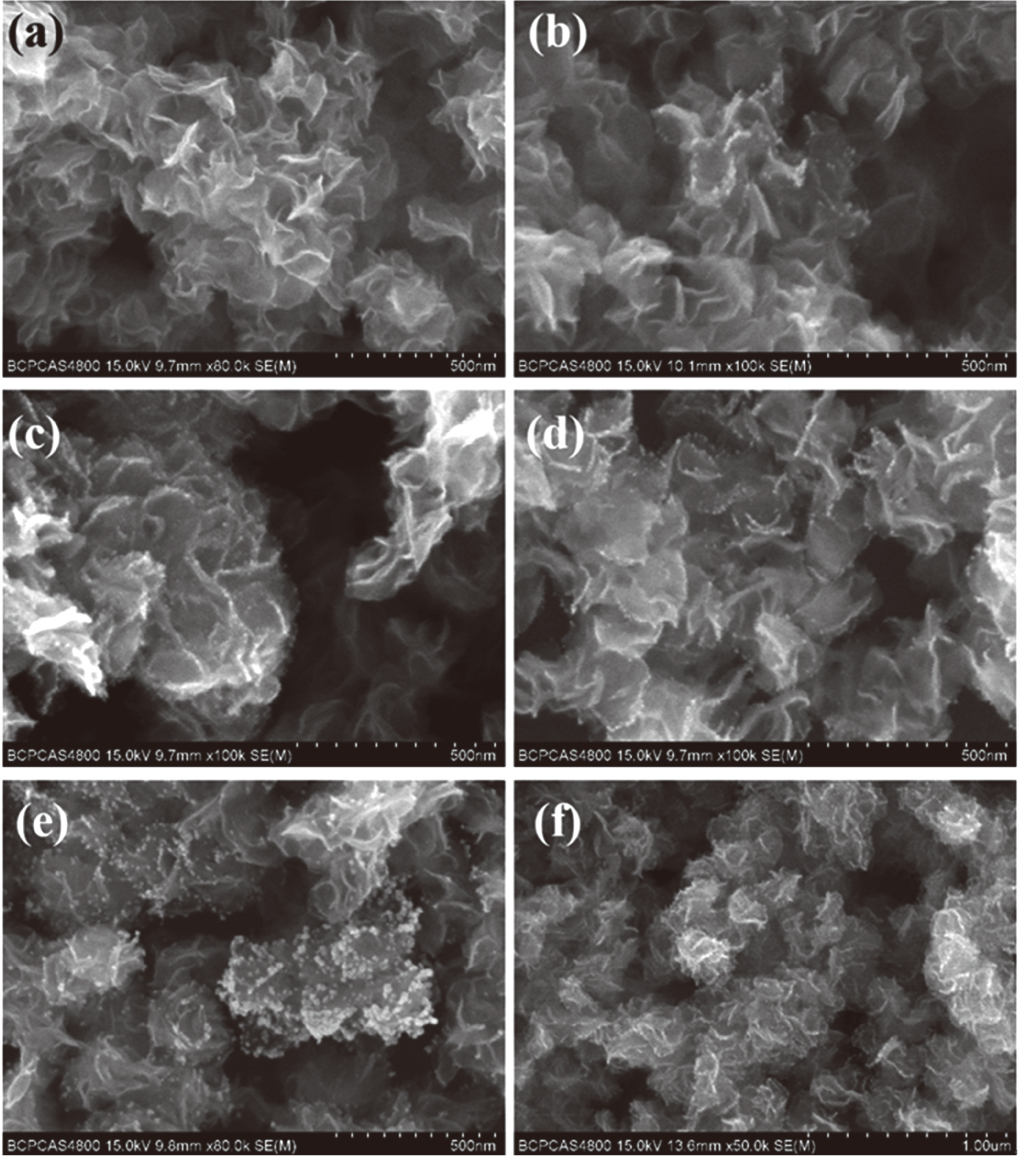 | Fig. 2. (color online) SEM images of (a) pure MoS2, (b) Au–MoS2-10, (c) Au–MoS2-25, (d) Au–MoS2-50, (e) Au–MoS2-100, and (f) Au–MoS2-150. |
The elemental mapping images based on Fig.
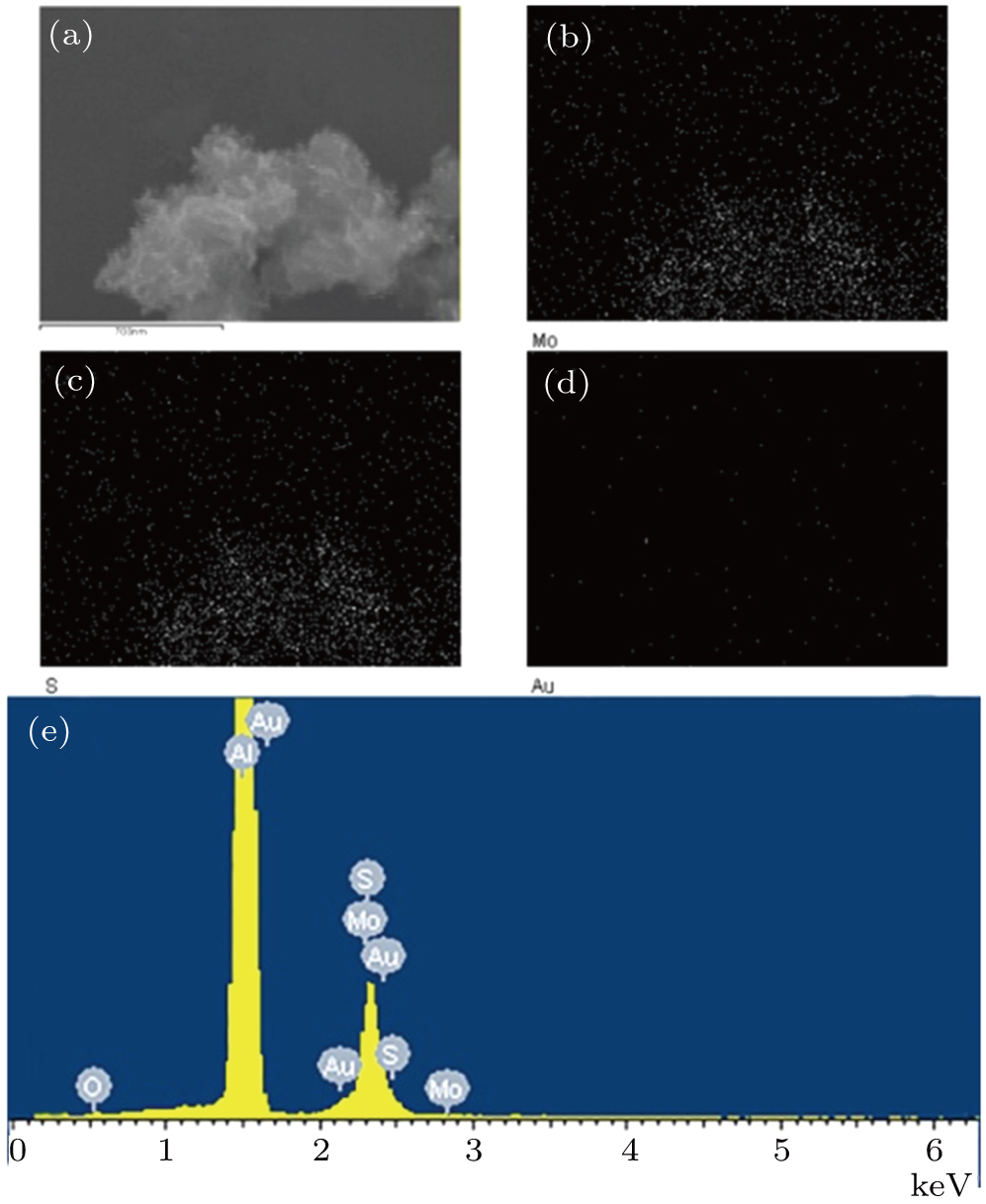 | Fig. 3. (color online) EDS elemental mapping images of (a) Au–MoS2-100 composite, (b) Mo, (c) S, (d) Au, and (e) energy dispersive x-ray spectrum of Au–MoS2-100 composite. |
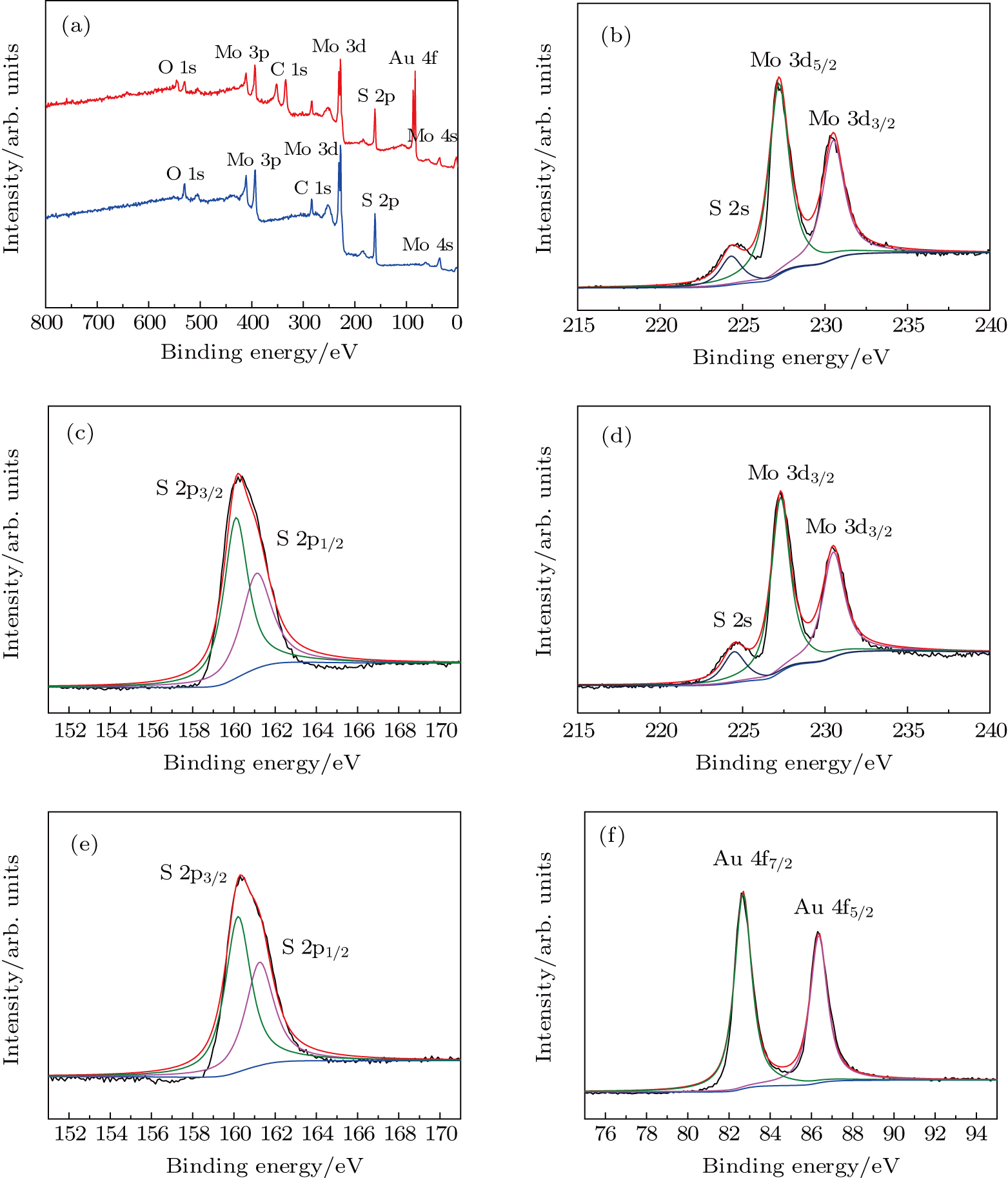
To explore the composition and chemical bonding configuration, XPS spectra of MoS2 are recorded before and after the introduction of Au. The XPS survey spectra of samples are shown in Fig.
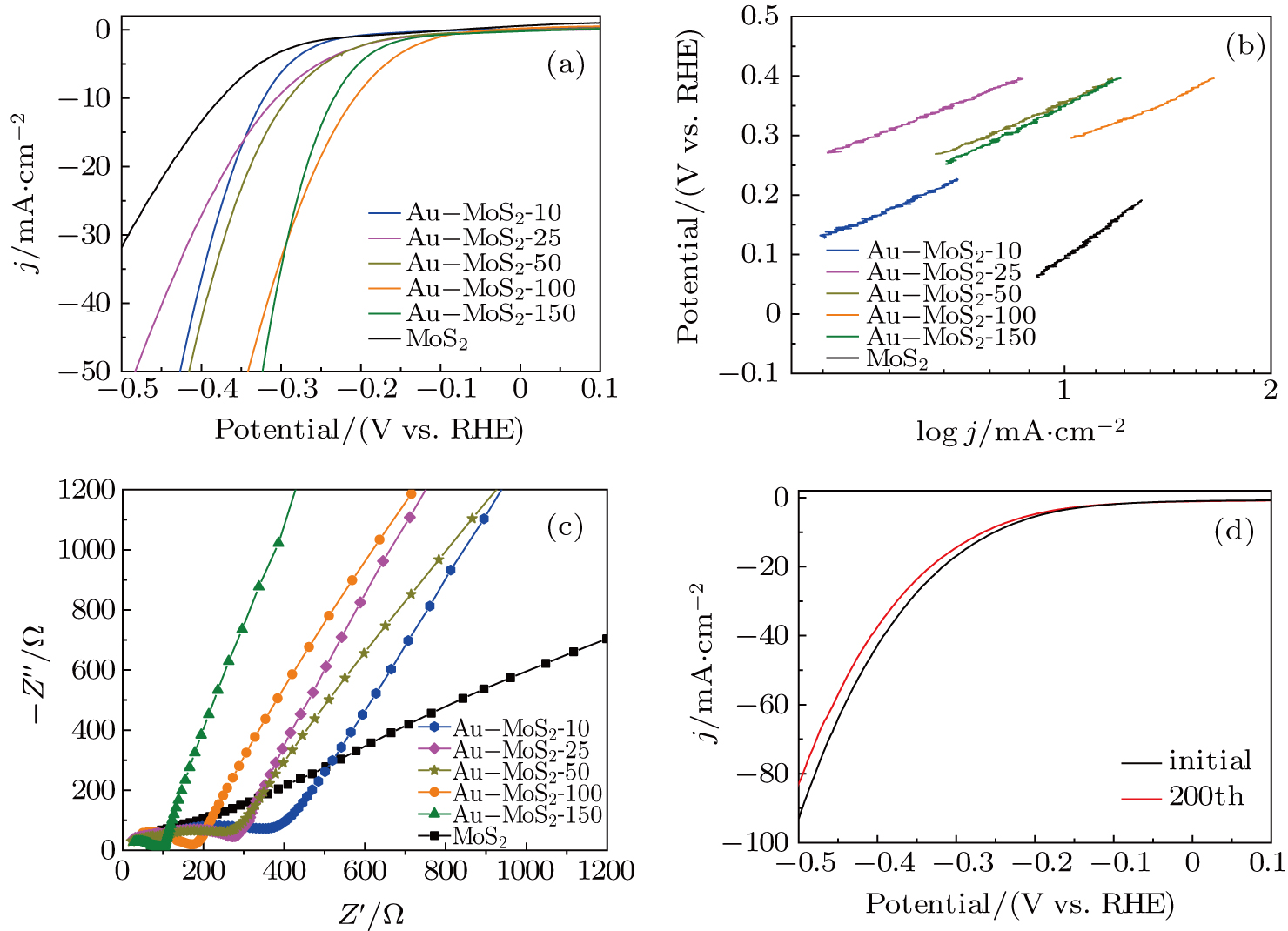
In order to evaluate the electrocatalytic HER activities of the Au–MoS2 composites, glassy carbon electrodes modified with pure MoS2, Au–MoS2-10, Au–MoS2-25, Au–MoS2-50, Au–MoS2-100, and Au–MoS2-150 compositions are prepared for polarization curves in 0.5-M H2SO4 electrolyte. As shown in Fig.
The Tafel slope is an intrinsic nature of the electrocatalyst material. The Tafel slope derived from the polarization curves is shown in Fig.
Composites of Au–MoS2 have been prepared by a solvothermal method. Au nanoparticles are in situ grown on the surface of a MoS2 nanoflower. The hydrogen evolution activity of the composite can be adjusted by the number of AuNPs. The Au–MoS2-100 shows excellent catalytic activity for hydrogen production with a lowest onset overpotential of 120 mV and a smallest Tafel slope of 163 mV·dec−1. The outstanding performance of the Au–MoS2-100 catalyst should be ascribed to the large specific surface area, large number of active sites, as well as more excellent ability to transfer electrons. Thus, the Au–MoS2 composite is a good candidate for advanced HER electrocatalyst in practical applications.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] |