Project supported by the National Natural Science Foundation of China (Grant Nos. 91733301, 51761145042, 91433205, 11474333, 51421002, 51627803, and 51572288) and the International Partnership Program of the Chinese Academy of Sciences (Grant No. 112111KYSB20170089).
Project supported by the National Natural Science Foundation of China (Grant Nos. 91733301, 51761145042, 91433205, 11474333, 51421002, 51627803, and 51572288) and the International Partnership Program of the Chinese Academy of Sciences (Grant No. 112111KYSB20170089).
† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 91733301, 51761145042, 91433205, 11474333, 51421002, 51627803, and 51572288) and the International Partnership Program of the Chinese Academy of Sciences (Grant No. 112111KYSB20170089).
A study of the self-healing phenomenon of Cu2ZnSn(S, Se)4 (CZTSSe) solar cells has shown more than 10% enhancement in cell performance after storage at room temperature for a week, with a significant improvement in the open-circuit photovoltage (Voc) and fill factor (FF). In addition, up to 10.45% power conversion efficiency (PCE) has been achieved. No obvious change in crystallinity, crystal phase, optical absorption or elemental distribution in the CZTSSe films was detected on examining the x-ray diffraction (XRD) pattern, Raman spectrum, ultraviolet-visible (UV-Vis), and TOF-SIMS. Further investigations on the charge carrier concentration, charge radiative recombination, and band structure suggest that the enhancement in PCE stems mainly from a reduction in deep defects of the CZTSSe semiconductor film.
Recently, thin-film solar cells based on Cu2ZnSn(S, Se)4 (CZTSSe) light absorbers have attracted wide interest from the scientific as well as industrial community owing to their distinctive advantage of earth abundant composition and low cost. By substituting the In atoms of Cu(In,Ga)Se2 (CIGS) with Zn and Sn atoms a similar lattice structure is retained; therefore, CZTSSe is generally considered as a potential alternative to the conventional chalcopyrite solar cells.[1–3] As a multi-component system,[4] CZTSSe has a large variety of minor phase and lattice defects[5,6] and this complexity hinders an in-depth understanding of the system. Therefore, so far, the highest certified efficiency of this cell is 12.6%,[7] which is much lower than that of the CIGS cells.[8] Therefore, the predecessors made a lot of efforts to understand the CZTSSe system and improved its device efficiency.
During the development of CZTSSe, many important experimental phenomena have been discovered and explored, such as component controlling, Na introducing, and band structure optimization. First, it is the well-known phenomena that Cu-deficient and Zn-rich growth condition yield the highest solar cell efficiency, then subsequent theoretical and experimental studies have shown that this particular composition of CZTSSe can help to reduce deep defects and harmful secondary phases,[6,9] which in turn leads to adjustment of the proportion of the components as Cu/(Zn+Sn) ≈ 0.8 and Zn/Sn = 1.1 ∼ 1.3. Second, the fact that devices that use soda-lime glass (SLG) as a base have higher efficiency has attracted attention. Further investigation confirmed that the Na in SLG can diffuse into the absorber and promote crystal growth,[10] increase carrier concentration,[11] and provide grain boundary barriers.[12] These results inspired us to add sodium to the sodium-free flexible substrate and try other similar metal treatments.[13–15] Third, in the early years, a nonuniform Ga/In ratio was found to have a good effect on the CIGS cells. Further studies show that the gradient distribution of these elements can form a gradient band gap, increase light absorption and reduce back surface recombination.[16–18] This concept was also used in CZTSSe and achieved high conversion efficiency.[19,20] Exploring experimental phenomena and applying them in practice is the law of development in a scientific research process.
Here, the phenomenon that the efficiency of the CZTS device can spontaneously increase after storage was observed by many researchers,[21–24] but barely acted upon. We believe that this phenomenon is important and worthy of in-depth research. In this study, systematic experiments were performed to examine the self-healing effect and elucidate its mechanism. By the process of self-healing, the CZTSSe solar cells exhibit over 10% improvement in power conversion efficiency (PCE) after storage at room temperature for a week. This improvement is mainly displayed in a significant enhancement in Voc and FF, besides, up to 10.45% PCE has been achieved. It is found that, for the CZTSSe absorber layer, its trap density can be significantly reduced after the room-temperature storage whereas the crystallinity, crystal phase, element distribution show no obvious differences. Moreover, this reduction would improve the charge depletion of the CdS/CZTSSe junction and interface energy band bending that would increase the built-in electric field. Based on these positive effects, a self-healing mechanism within the CZTSSe system is suggested, wherein, lattice and atom relaxation or internal stress relaxation driven by the thermodynamics may be possible origins.
Cu (99.99%), Zn (99.99%), S powder (99.95%, Aladdin), 1,2-ethylenediamine (AR), 1,2-ethanedithiol (AR), cadmium sulfate (AR), and thiourea (AR) were purchased from Aladdin, Sn (99.8%) and Se powders (99.5%) were from Alfa Aesar, and ammonium hydroxide (AR) was from Sinopharm Chemical Reagent Co. Ltd. All the chemicals were directly used as received without further purification.
The CZTSSe precursor solution with molar ratios of Cu/(Sn+Zn) = 0.74 and Zn/Sn = 1.25 was prepared in accordance with our previous work.[25] Cu (1.65 mmol), Zn (1.23 mmol), Sn (0.98 mmol), S (4 mmol), and Se (0.4 mmol) were dissolved in 5-mL 1, 2-ethylenediamine and 0.5-mL 1, 2-ethanedithiol. The mixture was continuously stirred for 1.5 h at 90°C to give a clear yellow solution. The precursor solution was spin-coated at 5000 rpm onto Mo-coated soda lime glass and heated on a hot plate at 380 °C for 1 min. The same process was repeated several times to produce a precursor film of about 1.5-μm thickness which was then selenized under Se/N2 atmosphere at 550 °C in a graphite box for 15 min to get the required CZTSSe film.
An SLG/Mo/CZTSSe/CdS/ZnO/ITO/Ag solar cell was prepared by successively depositing a CdS buffer by chemical bath method, radio frequency (RF) magnetron sputtered ZnO combined with indium tin oxide (ITO) window layers, and evaporated Ag collection grid. The active area of each device for J–V measurement was 0.18 cm2.
For characterization, the as-prepared CZTSSe film or device was labelled as “x d” which represents samples of the same batch stored for x days, for example, “0 d” is the sample without storage and “7 d” is that of the same batch stored in dark conditions for 7 days. These 7-d samples were sealed by a vacuum compressor under a rough vacuum and stored at room temperature. The current density–voltage (J–V) characterization for the solar cells was performed under AM1.5 illumination (1000 W · m−2) using an Xe-based light source solar simulator (Zolix SS150A), calibrated by a standard Si reference cell. The x-ray diffraction (XRD) was performed by using an x-ray diffractometer with Cu Kα as the radiation source (Empyrean, PANaltical). Raman spectra were collected by a Raman spectrometer (HR800, Jobin Yvon), using a 532-nm excitation laser with a power of 0.1 mW. The compositional depth profile was obtained by secondary-ion mass spectroscopy (TOF-SIMS 5, Germany ION-TOF GmbH), samples were CZTSSe and standard CdS films on Mo/SLG, 20-kV ion energy and 13.7-nA ion current was employed for analysis throughout the film, 5-kV ion energy and 4-nA ion current was used to analyze a superficial zone of CdS. The capacitance–voltage (C–V) and drive-level capacitance profile (DLCP) characterizations were measured on an electrochemical workstation (Versa STAT3, Princeton). The C–V data was performed at 100 kHz and 50-mV alternating current (AC) excitation source with direct current (DC) bias ranging from 0.5 V to −1.0 V, and the DLCP measurement was performed on 100 kHz while changing the AC perturbation voltage from 20 mV to 140 mV and DC bias from 0.5 V to −1.0 V. The band gap of the samples was determined by UV-Vis-NIR spectra on UV-3600 spectrophotometer, Shimadzu and steady-state PL spectra were obtained from a fluorescence spectrometer (FLS920, Edinburgh Instruments) with the time integration mode. The film was excited by a 535-nm monochromatic light from a xenon lamp. The valence band information was investigated via x-ray photoelectron spectroscopy (XPS) (ESCALAB 250X, Thermo Fisher Scientific) using a monochromatic Al Kα source of energy 1486.6 eV. The samples were charge-neutralized using an in-lens electron source combined with a low-energy (1 eV) Ar+ flood source. The samples for XPS characterization were as follows: “CZTSSe” sample was a fresh absorber layer, “CdS” sample was standard CdS (CBD for 11 min) deposited on CZTSSe base, “CZTSSe/CdS” sample was ultrathin CdS (CBD for 2 min) depositing on CZTSSe, which allowed the x-rays to penetrate the thin CdS to investigate both the thin CdS layer and the CZTSSe layer.
The carrier distribution and electric field inside the cell were theoretically calculated by solving the Poisson’s equations charge conservation and continuity equations in the dark by a general device simulator Analysis of Microelectronic and Photonic Structures (AMPS-1D). Dielectric constant 8.1 for CZTSSe, 9 for CdS, 7.8 for ZnO; defect densities of 1017 cm−3 and 1016 cm−3 were the values used to calculate the band alignments for 0 d and 7 d.
As shown in Fig.
It is widely reported that the Na diffusion within the CZTSSe film, usually introduced by post-annealing,[27–29] could help to improve the device performance. Relevant contrast experiments were also carried out to resolve this influence. For comparison, the CZTSSe films were fabricated on the quartz substrate (without Na).[30] Time-dependent current–voltage (J–V) results of the quartz substrate-based cell as well as that of the general soda-lime glass (SLG) are given in Fig.
Table
| Table 1.
Statistical result of the electrical properties of the cells (65 cells). RS, RSH, A, and J0 are the series resistance, shunt resistance, ideality factor, and reverse saturation current determined from light J–V data referenced Steven S Hegedus method.[31] . |
For a CZTSSe solar cell, its charge recombination is influenced by many aspects, such as the crystallization, secondary phase, element distribution and defect density.[5,32,33] First, the XRD pattern and Raman spectra are used to study the possible phase evolution during the storage. As seen in Fig.
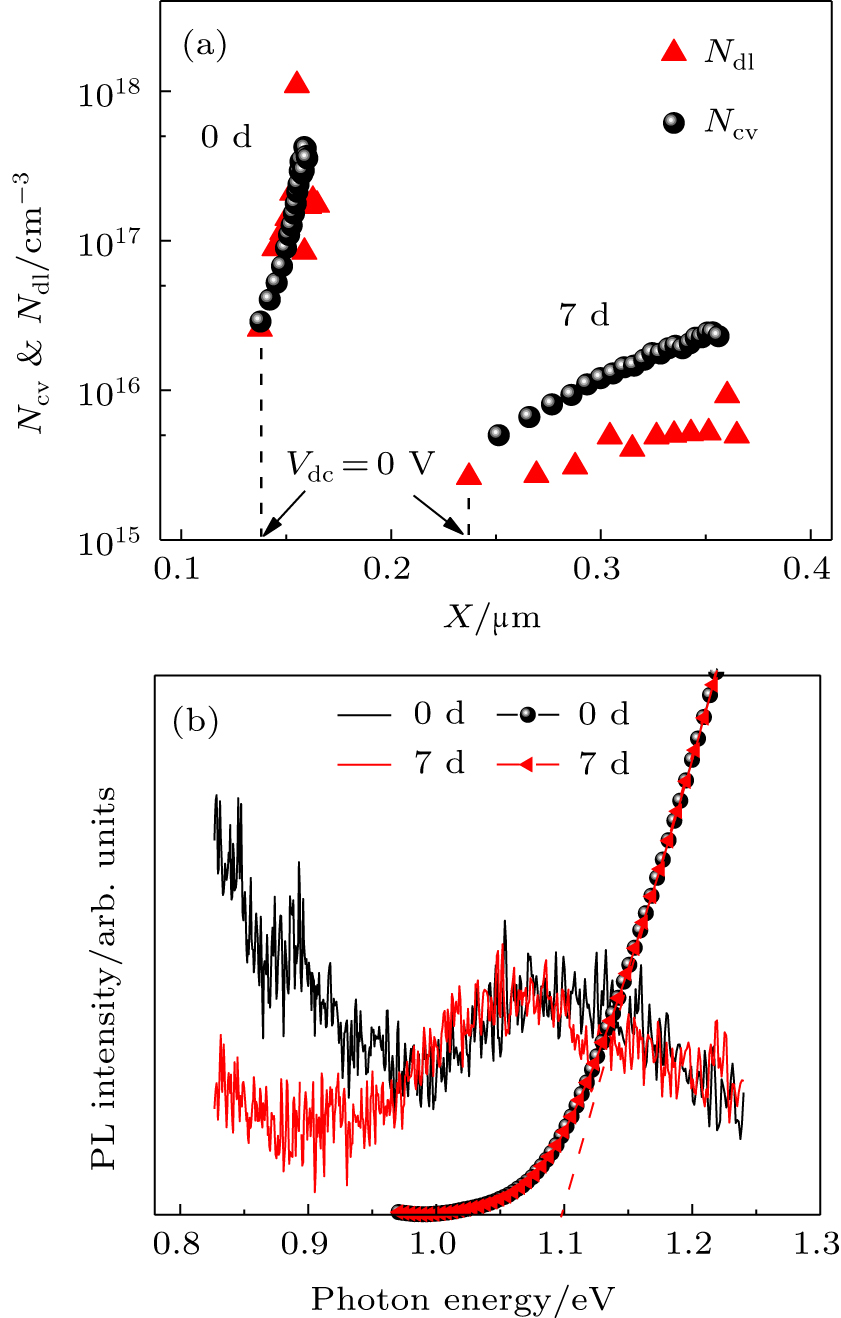
The DLCP and C–V measurements[43,44] have been conducted to evaluate defect properties of the CZTSSe absorber. To exclude the possible influence from other layers, the same batch of Mo/CZTSSe films is selected for the following measurement. In this batch, the samples are classified into two parts, defined as 0-d and 7-d samples, respectively, and one part was directly fabricated into the device without any storage while the other was stored for 7 days and then fabricated into the devices by following the same process. As shown in Fig.
Steady-state photoluminescence spectroscopy (PL) has been further employed to investigate bulk defects in the CZTSSe film. The CZTSSe film was covered by a PMMA layer (∼ 200 nm) to prevent the surface from the external atmosphere (such as oxygen and water). As shown in Fig.
To further confirm the above assumption, the influence of the reduction in the deep-defect density on the electric properties of the cell has been studied by theoretical simulation on the band alignment.[46,47] As shown in Fig.
In view of the above discussion, it is suggested that the deep defects, like Sn-related or S/Se vacancy defects, in the CZTSSe absorber layer are spontaneously reduced after storage at room temperature. This reduction could help to suppress charge recombination, and furthermore lead to a wider depletion and a stronger built-in electric field. Therefore, Voc and FF significantly increase after the self-healing and lead to better device performance. This improvement may be attributed to: i) the atomic relaxation in grain boundary (GB) regions.[48] The dangling bonds at the GB create localized defect states,[49,50] and the nanoscale horizontal diffusion of atoms is undetectable by SIMS, but can break or weaken the detrimental dangling bonds thus eliminating the deep gap states; ii) the release of internal stress within the CZTSSe film. The compressive internal stress in the asprepared film[51] is spontaneously released at room temperature,[52] which helps to optimize the spatial energy band distribution and thus reduce the charge localization centers. This self-healing effect presents a valuable opportunity to recognize the hidden mechanism that was limiting the device performance.
An impressive self-healing effect of the defects within the CZTSSe absorber is observed, which significantly contributes to the performance enhancement of the solar cell. No obvious changes are found in the crystalline, phase, element composition, or impurity atom diffusion of the CZTSSe film. Further investigation reveals that this effect is mainly assigned to a significant reduction in the deep defect density of the CZTSSe semiconductor, and the relevant physics mechanism behind this behavior has been investigated. Spontaneous passivation of dangling bonds at the grain boundaries and the release of local stress within the CZTSSe at room temperature could be possible reasons for this reduction in the deep defect density. This interesting self-healing effect and the physics mechanism involved therein provide an opportunity to further understand the performance-limited mechanisms of CZTSSe devices, which has great values for the device optimization and performance enhancement.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] | |
| [41] | |
| [42] | |
| [43] | |
| [44] | |
| [45] | |
| [46] | |
| [47] | |
| [48] | |
| [49] | |
| [50] | |
| [51] | |
| [52] |