Accurate quantification of hydration number for polyethylene glycol molecules
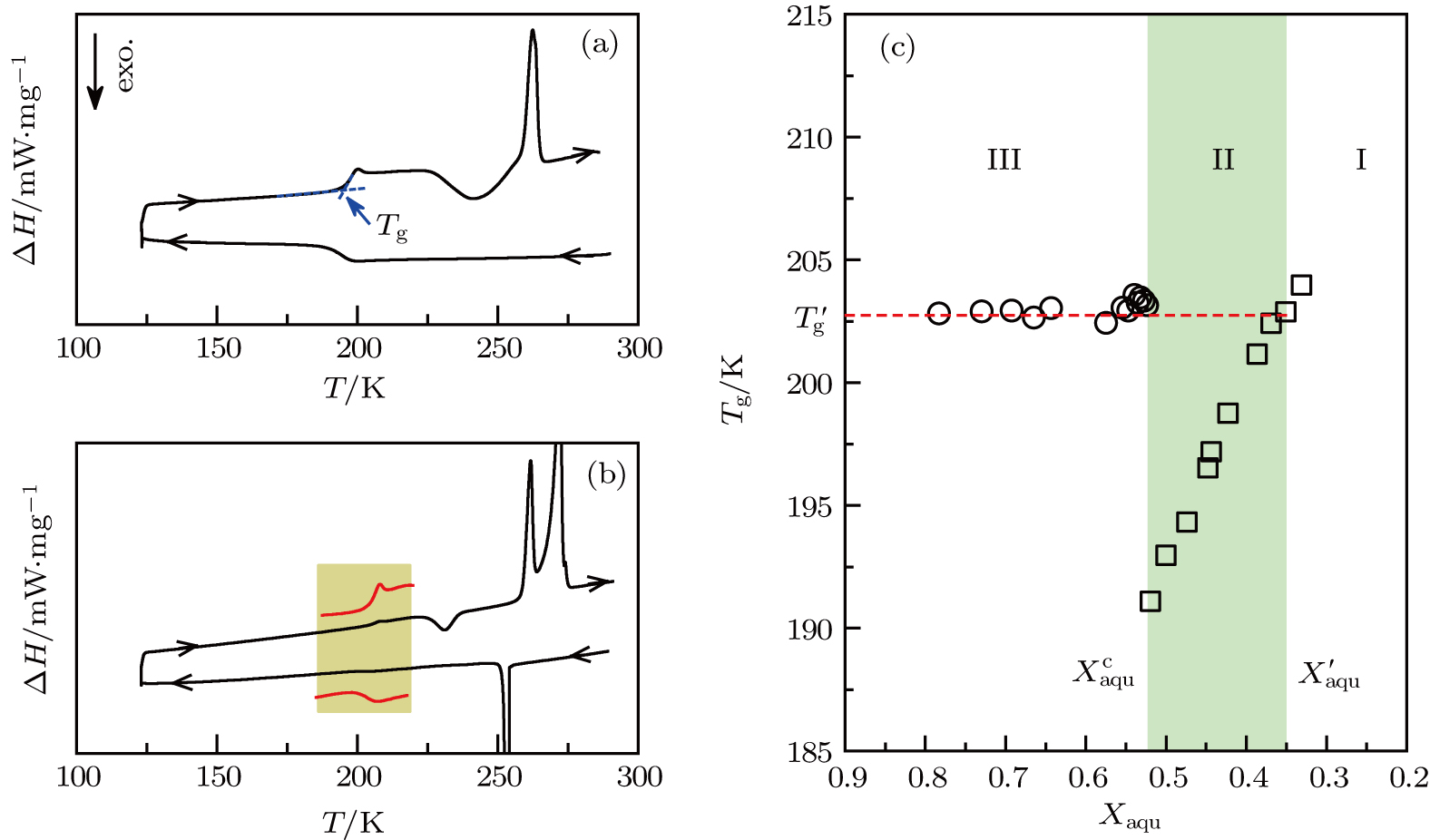
(color online) (a) DSC thermograms of aqueous solutions of PEG 20000 with a mass fraction of water of (a) Xaqu = 0.47, and (b) Xaqu = 0.78. Water in the high-concentration solution (a) vitrifies easily upon cooling, and upon the subsequent reheating the devitrified solution, a portion of water cold-crystallizes into ice (exothermic peak) which then melts upon further heating (endothermic peak). In the water-rich solution (b), water directly crystallizes into ice upon cooling, followed by vitrification of the freeze-concentrated phase. Upon subsequent reheating, the devitrified freeze-concentrated phase undergoes cold-crystallization first and then melts with further increasing temperature. Ice precipitated upon the cooling process melts at a comparatively higher temperature. (c) Water content dependence of glass transition temperature Tg for the high-concentration solutions (square) and for the freeze-concentrated phase (circle) resulting from icing of the water-rich solutions, respectively. At