† Corresponding author. E-mail:
Nasicon materials (sodium superionic conductors) such as Li1.5Al0.5Ge1.5(PO4)3 (LAGP) and Li1.4Al0.4Ti1.6(PO4)3 (LATP) have been considered as important solid electrolytes due to their high ionic conductivity and chemical stability. Compared to LAGP, LATP has higher bulk conductivity around 10−3 S/cm at room temperature; however, the apparent grain boundary conductivity is almost two orders of magnitude lower than the bulk, while LAGP has similar bulk and grain boundary conductivity around the order of 10−4 S/cm. To make full use of the advantages of the two electrolytes, pure phase Li1.5Al0.5Ge1.5(PO4)3 and Li1.4Al0.4Ti1.6(PO4)3 were synthesized through solid state reaction, a series of composite electrolytes consisting of LAGP and LATP with different weight ratios were designed. XRD and variable temperature AC impedance spectra were carried out to clarify the crystal structure and the ion transport properties of the composite electrolytes. The results indicate that the composite electrolyte with the LATP/LAGP weight ratio of 80:20 achieved the highest bulk conductivity which shall be due to the formation of solid solution phase Li1.42Al0.42Ge0.3Ti1.28(PO4)3, while the highest grain boundary conductivity appeared at the LATP/LAGP weight ratio of 20:80 which may be due to the excellent interfacial phase between Li1+xAlxGeyTi2−x−y(PO4)3/LATP. All the composite electrolytes demonstrated higher total conductivity than the pure LAGP and LATP, which highlights the importance of heterogeneous interface on regulating the ion transport properties.
Lithium ion batteries have been widely used. All kinds of applications require high safety and high energy density. The electrolytes used in current commercial lithium ion batteries are flammable organic liquid electrolytes, which leads to thermal runaway. To enhance the safety and further improve the energy density of lithium batteries, researchers have paid wide attention to develop the solid state electrolyte which could be inflammable and compatible with the Li-contained high capacity anode.[1,2]
Generally, the solid state electrolytes are divided into two types. One is the polymer electrolyte, which uses the polymer molecular like polyethylene oxide (PEO), polyvinyliene fluoride (PVDF), polymethyl methacrylate (PMMA), polyacrylonitrile (PAN), polysiloxane, and so on, as the skeleton materials to cooperate with the lithium salt forming the dry polymer electrolyte.[3] These polymer electrolytes usually have low room temperature ionic conductivity in the vicinity of 10−6 S/cm, which is nearly three orders of magnitude lower than that of the liquid electrolytes and could not satisfy the kinetic demands of real applications in ambient surroundings. The other is the inorganic solid state electrolyte, mainly including sulfide and oxide compounds.[4] Usually, the sulfide electrolyte has higher ambient ionic conductivity than the oxide electrolyte, however, most sulfide electrolytes are not stable in the air and the chemical stability is relatively poor.[5] Compared to the sulfide electrolyte, the solid oxide electrolyte has better chemical and electrochemical stability. Some of them have acceptable room temperature ionic conductivity. The most interesting materials are garnet structure Li7La3Zr2O12 (LLZO),[6] perovskite structure Li0.5La0.5TiO3 (LLTO),[7] LISICON structure Li14Zn(GeO4)4 (LZGO),[8] NASICON structure Li1.5Al0.5Ge1.5(PO4)3 (LAGP),[9] Li1.4Al0.4Ti1.6(PO4)3 (LATP), and so on.[10,11]
Compared to LAGP and LATP, LLZO and LLTO contain rare earth elements, which could be costly. Besides, LLZO is not very stable in moist air while LLTO has a large grain boundary resistance.[12] The LISICON type LZGO has very low room temperature ionic conductivity around 10−7 S/cm.[1] LAGP and LATP possess acceptable ionic conductivity. The bulk conductivity of LATP can be as high as 10−3 S/cm at room temperature. However, the grain boundary conductivity of LATP is nearly two orders of magnitude lower than the grain conductivity, generally of the order of 10−5 S/cm.[13]
In order to improve the grain boundary ion transport properties and the total ionic conductivity, researchers have made great efforts. Different preparation methods have been tried, including sol–gel, solution methods, pechini synthesis, melt quench, hydrothermal synthesis, co-precipitation, and mechano-chemical. The heat treatment processes have been investigated, including cold sintering, dry pressing, low temperature pressing, and varying calcination conditions.[14–21] Doping has also been widely studied.[22–24] Most of the above methods have improved the total conductivity to some extent and can enhance the grain boundary ionic conductivity. However, the improvement is not very significant.
Another route is to introduce the second phase into the pure LATP to modify the boundary, such as the ion insulator AlPO4, Al2O3, B2O3, MgO, Li2O,[25–29] or high dielectric constant materials like BaTiO3, SrTiO3,[28] and some poor lithium ion conductors with relative low melting point like Li2CO3, Li3PO4 as the sintering agent.[30] These modifications do improve the grain boundary conductivity and increase the total conductivity, which could be caused by high compactness or high carrier concentration in the grain boundary. However, the effect is still not very significant. It is found that the LATP/LAGP bi-layer electrolyte or LLTO/LATP composite electrolyte received significant effect on improving the total conductivity,[31,32] but the enhancement mechanism is not very clear and requires further investigation by using impedance spectra at a wide temperature range.[13]
Inspired by the previous efforts, in this paper, a similar but not well studied strategy is introduced. The LAGP is chosen as the second phase for the LATP due to its high grain boundary conductivity of 10−4 S/cm. It is almost one order of magnitude higher than that of the LATP. The main contents in this paper include: 1) solid state synthesis of the pure phase LAGP and LATP, 2) solid state synthesis of a series of composite electrolytes with LATP and LAGP by adjusting the weight ratio of the two electrolytes, 3) preparation and optimization of the ceramic sheets, 4) measurements of variable temperature AC impedance spectroscopy, 5) detailed data analysis and quantitative conclusion.
The aluminum doped Li1.5Al0.5Ge1.5(PO4)3 (LAGP) was prepared through solid state reaction. The stoichiometric mixtures of GeO2, (NH4)2HPO4, Li2CO3, and Al2O3 were mixed for 1 hour in an agate mortar. The mixtures were then heated at 400 °C for 2 hours with a heating rate of 2 °C/min in air to release the volatile products. The heated mixtures were ball-milled for 8 hours and then pressed into pellets in a stainless steel die and sintered at 900 °C for 3 hours with a heating rate of 2 °C/min in air.
The aluminum doped Li1.4Al0.4Ti1.6(PO4)3 (LATP) was prepared similarly like preparing the LAGP. The differences lie in the original materials and the sintering process. The stoichiometric mixtures of TiO2, NH4H2PO4, Li2CO3, and Al2O3 were mixed for 1 hour in an agate mortar. The mixtures were then heated at 90 °C with a heating rate of 1 °C/min, then heated to 200 °C with a heating rate of 0.5 °C/min, finally heated to 400 °C with a heating rate of 0.5 °C/min to release the volatile products. The heated mixtures were ball-milled for 8 hours and then pressed into pellets in a stainless steel die and sintered at 900 °C for 5 hours with a heating rate of 2 °C/min in air.
After synthesizing the pure phase LAGP and LATP, the powders of LAGP and LATP and a series of composite electrolytes with different LATP/LAGP weight ratios (labeled as pure LATP, 80:20, 50:50, 20:80, and pure LAGP) were pressed into sheets with a diameter of 10 mm and sintered at different temperatures from 750 °C to 950 °C with a 50 °C interval for 3 hours under a heating rate of 2 °C/min in air.
The phase purity of the LAGP and LATP powders as well as the composite electrolytes that were sintered at 900 °C was examined at room temperature using a Bruker D8 Advance diffractometer with Cu-Kα radiation and a LYNXEYE detector. Data was taken in the range of 10°–60° with 0.02° per step and a count time of 0.2 s at each step.
AC impedance measurement was carried out using a NOVO control analyzer. The gold was sputtered on both sides of the ceramic sheet as the block electrodes. The testing frequency range was from 1 Hz to 10 MHz and the temperature range was from 233 K to 293 K with a step of 10 K. The temperature was held for 30 min before each measurement.
The XRD patterns of Li1.5Al0.5Ge1.5(PO4)3 and Li1.4Al0.4Ti1.6(PO4)3 are presented in Fig.
 | Fig. 1. (color online) XRD patterns of (a) Li1.5Al0.5Ge1.5(PO4)3 and (c) Li1.4Al0.4Ti1.6(PO4)3, PDF cards for (b) LiGe2(PO4)3 and (d) PDF card LiTi2(PO4)3. |
The impedance spectroscopy of LAGP and LATP measured at 233 K is shown as the Nyquist plot in Fig.
 | Fig. 2. (color online) Impedance data presented in Nyquist plot for (a) Li1.5Al0.5Ge1.5(PO4)3 and (b) Li1.4Al0.4Ti1.6(PO4)3 sintered at 900 °C for 3 hours. |
Figure
 |
 | Fig. 3. (color online) Relationship of relative density and apparent grain boundary conductivity of Li1.5Al0.5Ge1.5(PO4)3 with the sintering temperature. |
From Fig.
Figure
Figure
 |
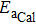
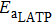

Figure
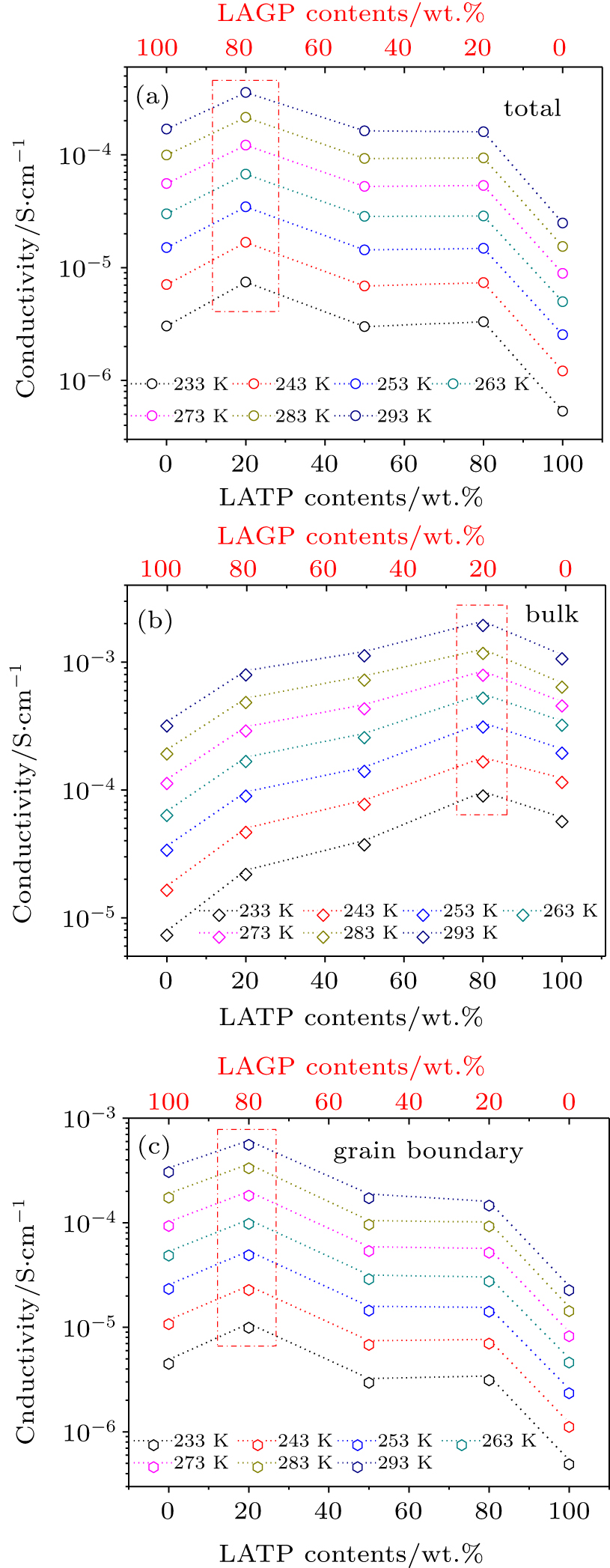 | Fig. 5. (color online) Conductivity of different electrical regions in the composite samples at different temperatures: (a) total conductivity, (b) bulk conductivity, (c) grain boundary conductivity. |
Combining the results of Figs.
Suppose that the composite electrolytes consists of electrolyte A and electrolyte B with different intrinsic bulk conductivities σA and σB, the volume ratio or weight ratio of electrolyte A is x 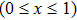
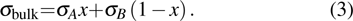 |
Figure
 |
 |
 | Fig. 6. (color online) (a) Relationship of response frequency with response angle for different composite electrolytes and (b) relationship of conductivity with response frequency. |
Figure
 |
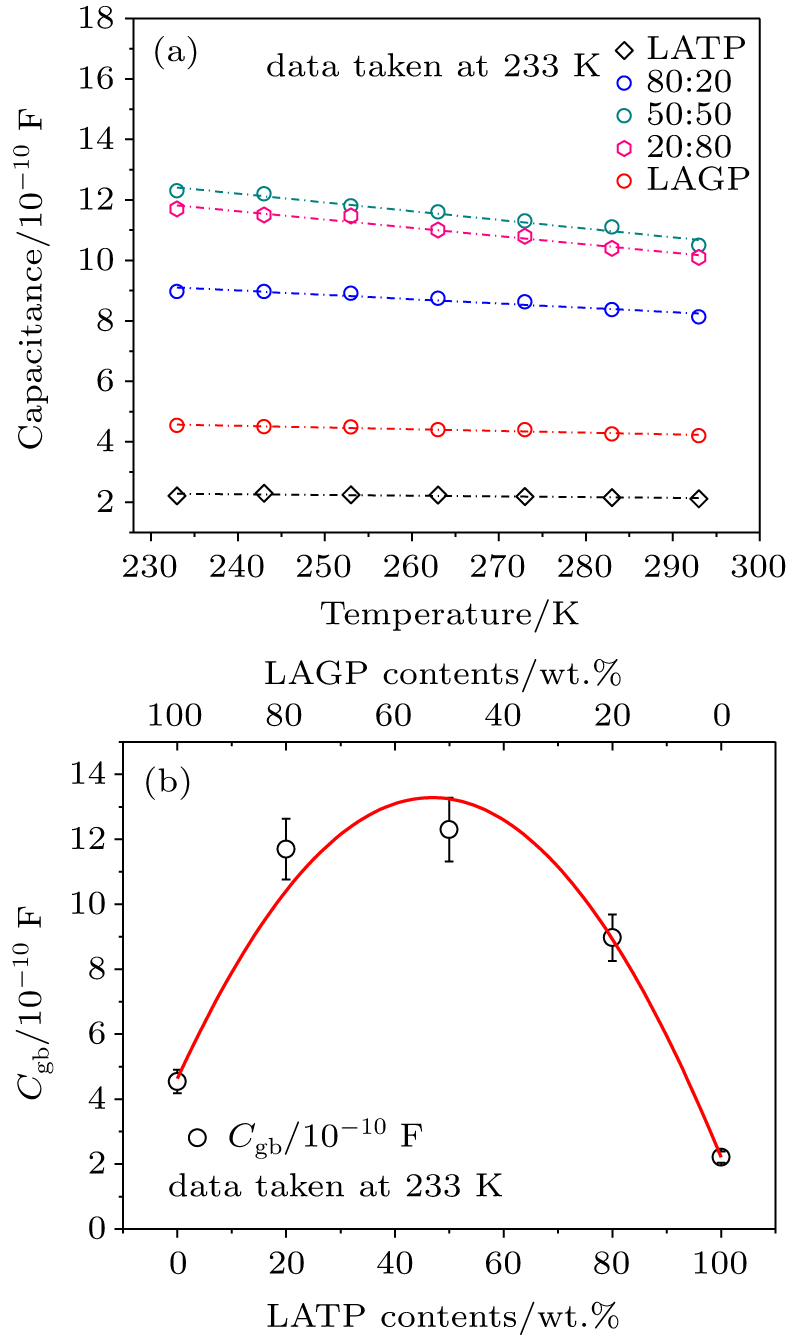 | Fig. 8. (color online) (a) Grain boundary capacitance of different composite samples and (b) the relationship of grain boundary capacitance with ratio of LATP to LAGP. |
Figure
Figure
| Table 1.
Geometry parameters of the composite electrolytes. . |
In fact, the grain boundary conductivity is closely related to the compactness of the ceramic sheet, which has been reported in the previous study.[13] According to table
For the bulk conductivity enhancement mechanism, which has been discussed in the above paragraph, the compactness has very little impact on the accurate measuring of the bulk conductivity, as illustrated in Fig.
The XRD patterns of the composite electrolytes sintered at 900 °C for 3 hours are displayed in Fig.
Pure phase LAGP and LATP were synthesized through solid reaction and a series of composite electrolytes consisted of LAGP and LATP with different weight ratios were designed. Systematic variable temperature AC impedance spectra were investigated to clarify the ion transport properties of the composite electrolytes. The results indicate that the composite electrolyte with the LATP/LAGP weight ratio of 80:20 has the highest bulk conductivity, which may be due to the solid solution phase of Li1.42Al0.42Ge0.3Ti1.28(PO4)3, while the highest grain boundary conductivity appears at the LATP/LAGP weight ratio of 20:80 and this shall be due to the excellent interfacial phase between Li1+xAlxGeyTi2−x−y(PO4)3/LATP, which can acquire high grain boundary conductivity at a relatively low compactness. All the composite electrolytes have higher total conductivity than the pure LAGP and LATP, which highlights the importance of fine tuning the heterogeneous interface on designing a composite fast ionic conductor. Finally, to further comprehend the micro mechanism of the enhanced ionic conductivity at the interface, future research work is on the way to reveal the interfacial structure from the atomic level.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] |




