† Corresponding author. E-mail:
By using classical ensemble method, we investigate the double ionization of C3H6 molecule with different structures (propene and cyclopropane) in intense laser fields. The numerical results show that the non-sequential double ionization occurs in propene molecule rather than cyclopropane molecule in 1200 nm laser field. To further explain this interesting phenomenon, the momentum distribution of double ionized electrons is presented and the result presents the “finger-like” structure at about 30 TW/cm2 of propene molecule, and this structure is more obvious than that in cyclopropane molecule. The above phenomena are also demonstrated by analysing the energy distributions of double-ionized electrons versus time. Moreover, we also investigated the angular distribution at the end of pulse, which is different between propene and cyclopropane.
The physical sight of double ionization processes of atoms and molecules has attracted both practical and fundamental interest.[1–3] Dynamics of simple systems has been studied thoroughly both theoretically and experimentally.[4,5] Corkum[6] proposed a rescattering model that one electron ionizes first and revisits the core to let the second electron free by collision, which is widely accepted to explain the non-sequential double ionization (NSDI). With the development of laser technology, the tracing of electron motion in atoms and molecules in strong-field also has been a hot topic recently and its physical insight still needs further exploration.[7,8] Recently, it turns out that semi-classical or classical simulations are useful to treat the strong-field ionization with very strong two-electron correlation, such as NSDI.[9–14,21]
Comparing with atoms, molecules exhibit much more complicated processes in strong-field ionization, owing to their diverse molecular structure and additional nuclear degree of freedom. The underlying mechanism of molecular double ionization (DI) is still not clearly understood, which stimulates further investigations on the topic. There exists evidence that has shown that NSDI is also contributed to DI of molecules when subjected to strong laser fields. The “knee” structure, which is viewed as a signature of NSDI of atoms, has been observed in simple diatomic[15–18] and linear triatomic molecules,[19–21] and even more complicated polyatomic molecules.[22] Bandrauk et al.[23,24] also investigated the HHG and ionization process of symmetric and unsymmetric molecules. Moreover, the bichromatic counterrotating circularly polarized laser fields attracts a lot of attention and there have many excellent works on the dynamics of atoms and molecules in the bichromatic counterrotating circularly polarized laser fields.[25–30]
Thus, in this paper, we will investigate the double ionization mechanism of C3H6 with different structures and compare them together. We will illustrate how non-sequential double ionization of propene happens at 1200 nm intense laser fields. Particularly, we will investigate the corresponding momentum and energy distributions of doubly ionized electrons to study the DI process of propene and cyclopropane molecule.
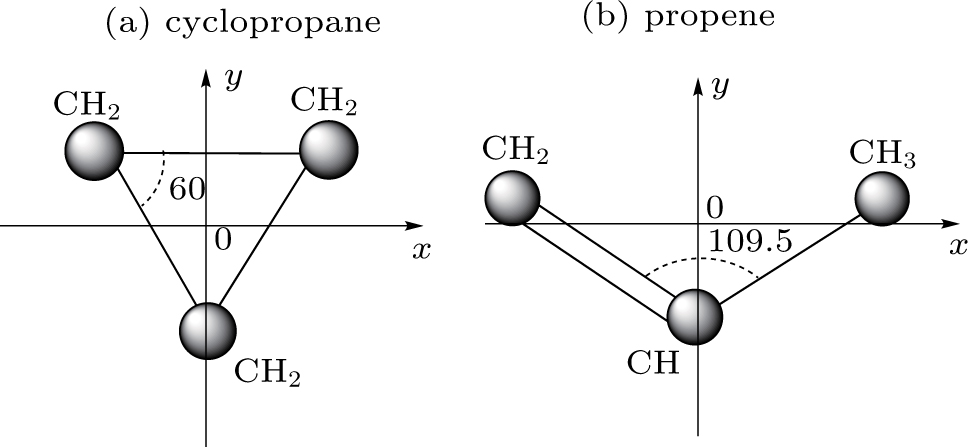
In this paper we use the classical ensemble method, which has previously been used[11,12,21] successfully to explore the ionization dynamics of atoms and three-atom molecule CS2[31] in intense laser fields. In C3H6 with different structures, the bond angle between C–C–C are 109.5 degree and 60 degree in propene and cyclopropane, respectively. The structure of cyclopropane is shown in Fig.
Propene, also known as propylene or methyl ethylene, is an unsaturated organic compound having the chemical formula C3H6, which structure is shown in Fig.
In our calculation, the coordinate of mass center is used and the classical Hamiltonian of C3H6 molecules in an intense laser field can be given by (atomic units are used throughout unless otherwise stated)
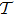
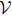
The canonical system of equations for CS2 molecule is
The symplectic method is the difference method that preserves the symplectic structure and especially suitable for the long-time many-step calculations. We choose a set of initial stable states 
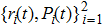
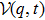
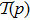
In our calculation, we assume that the initial condition has the same energy approximately equal to the sum of first and second ionization energy of C3H6 molecule (0.7864 for cyclopropane and 0.7945 for propene). Comparing with the molecular structure of the hydrogen molecule (0.5668 for H2, 0.56837 for D2, and 0.56756 for HD),[32] the solution of C3H6 molecule is a little bit bigger than the ionization energy of hydrogen molecules. Besides, many researchers have obtained values for the dissociation energies of C3H6 molecule (0.3748 for cyclopropane and 0.3546 for propene ) and of hydrogen molecule (0.5393 for H2, 0.5423 for D2, and 0.5406 for HD) by comparison.[32,33] Obviously, the dissociation energies of C3H6 molecule is a little bit smaller than hydrogen molecules. We defined the energy of each electron as E1(t) and E2(t), respectively. If both E1(t) and E2(t) are greater than zero at the end of the laser pulse, the double ionization occurs.
We investigate the ionization process of the C3H6 molecule in intense laser field. The linearly polarized electric field is chosen as 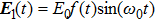
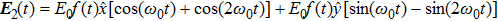
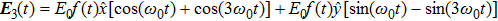

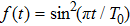
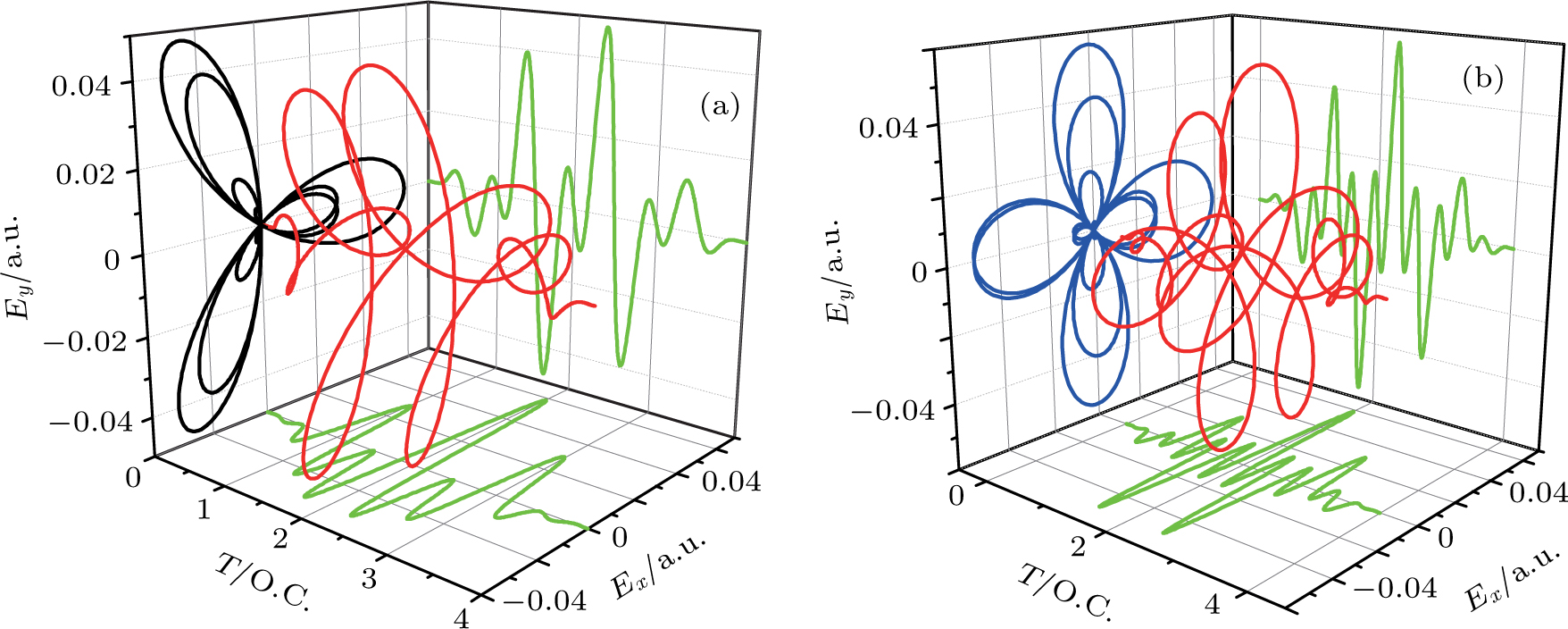 | Fig. 2. (color online) The frame of counter-rotating laser fields with ω +2ω and ω +3ω, respectively. The laser wavelength is 1200 nm. |
Figure
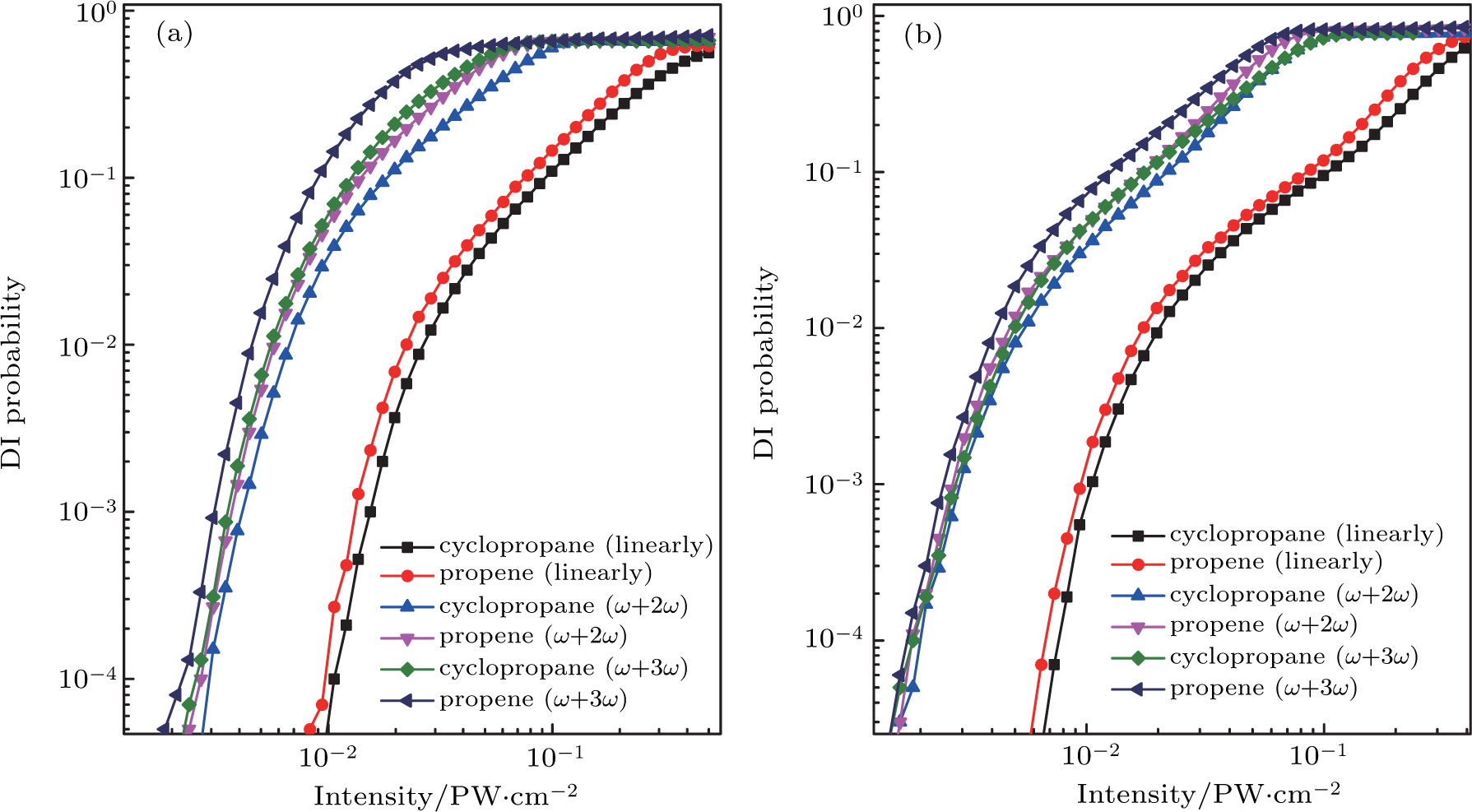 | Fig. 3. (color online) The double ionization probability of C3H6 with the structure of cyclopropane and propene as a function of laser intensity in (a) 800 nm and (b) 1200 nm intense laser fields. |
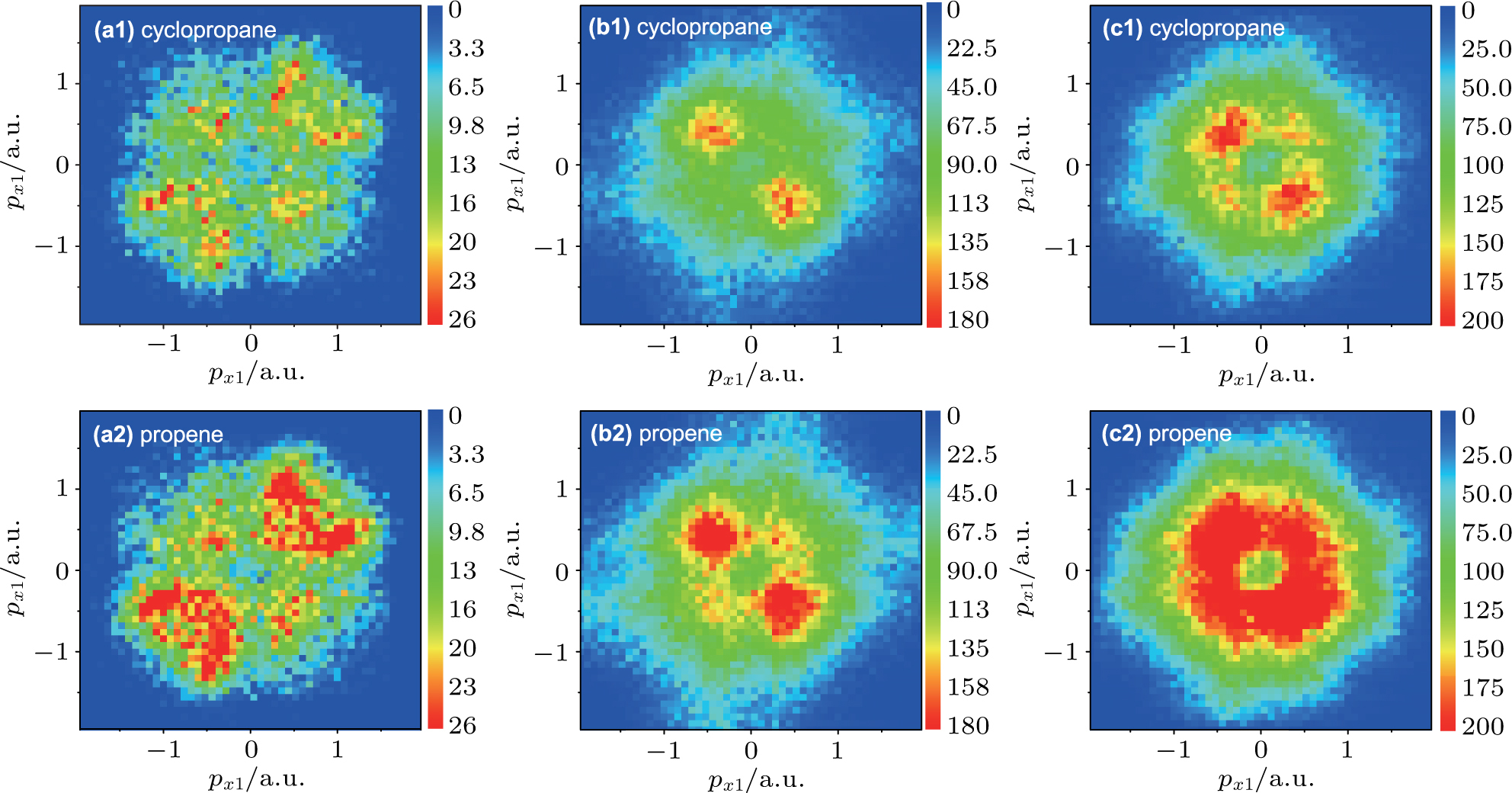
In order to further understand or identify whether the non-sequential double ionization mechanism of C3H6 molecule exists, we calculated the corresponding momentum distribution of correlated electrons of C3H6 molecule with the structures of cyclopropane and propene at the laser intensity 30 TW/cm2, shown in Fig.
The classical limits lead to a well-known cutoff laws: for direct above-threshold ionization (ATI), where the electron once ionized does not significantly interact anymore with the ion, its maximum energy is 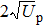
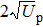
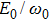
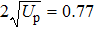
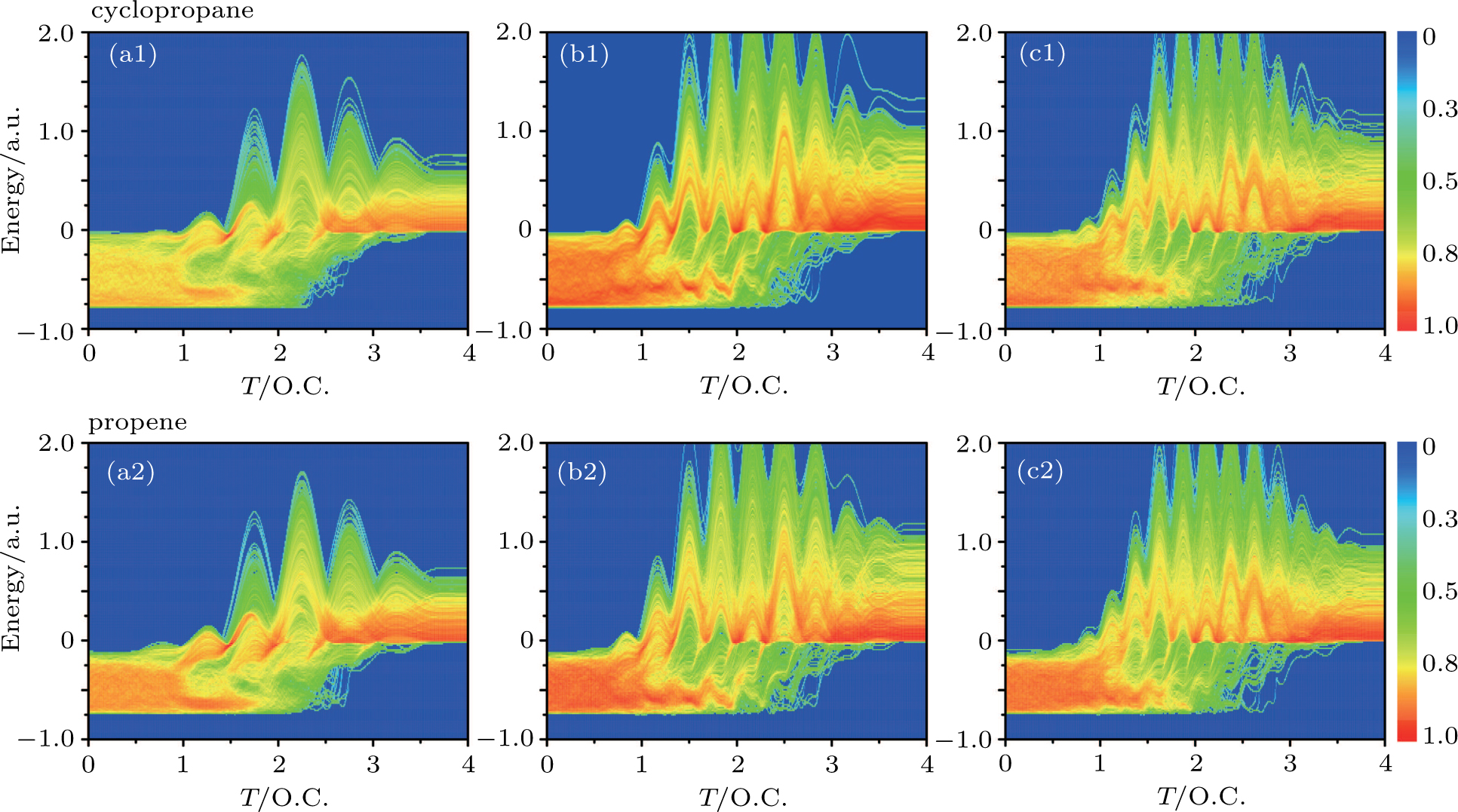
In the classical ensemble approach, it is convenient to investigate NSDI and DI by tracing the classical energy trajectories. In order to reveal the DI dynamics of C3H6 molecule, we take into account all the two-electron energy trajectories that lead to DI events and make statistics at every moment of the time evolution, and furthermore we choose the recollision trajectories from them. Then the energy distribution of DI electrons as a function of time in intense laser fields can be obtained, as shown in Fig.
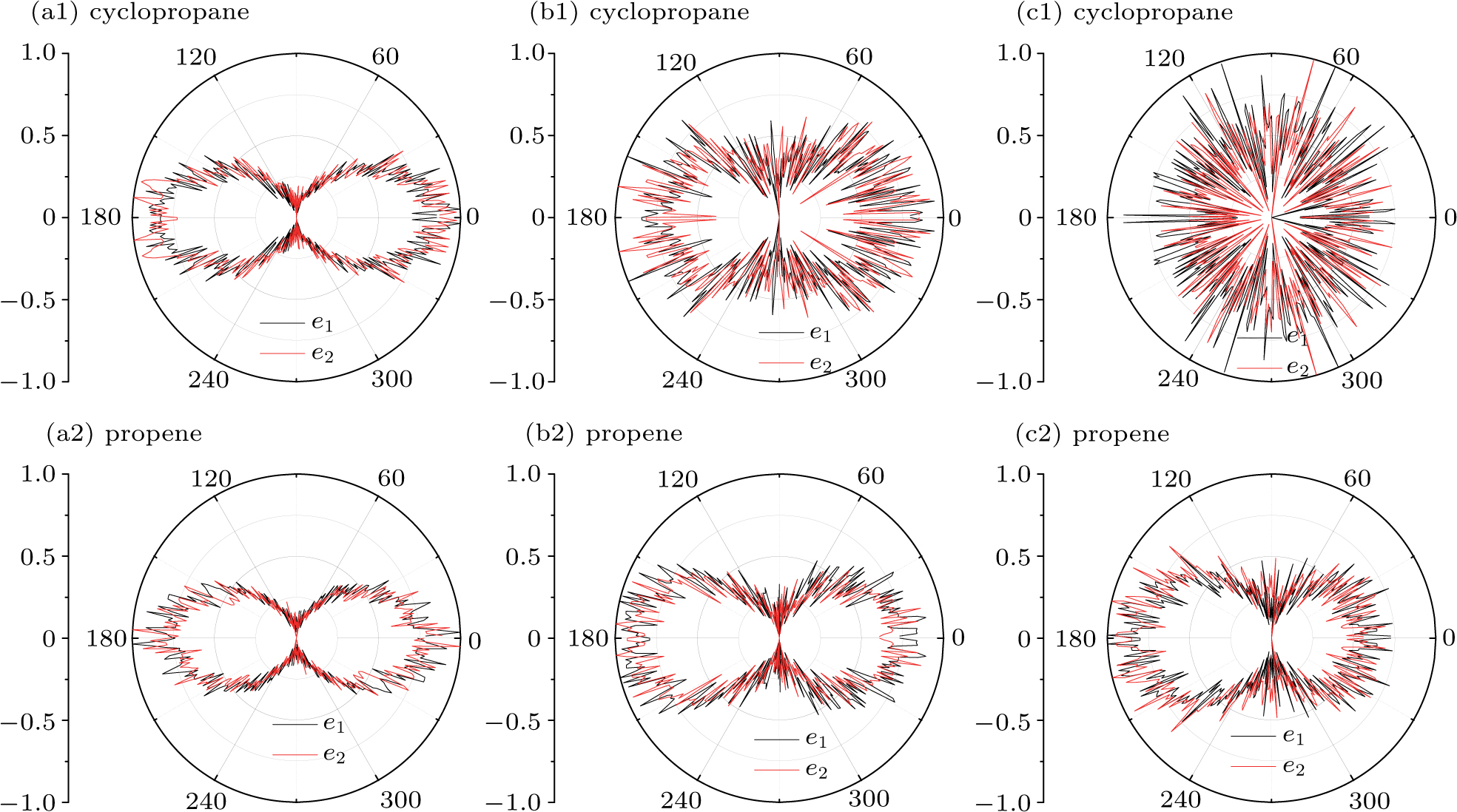
In addition, we also calculate the angular distributions at the end of pulse of propene and cyclopropane in Fig.
We performed a theoretical study on strong-field DI process of C3H6 molecule with the structure of cyclopropane and propene in 800 nm and 1200 nm laser fields. A “knee” structure occurs in the DI yield in 1200 nm linearly polarized laser fields and counter-cotating laser fields, showing the evidence of NSDI in strong-field ionization of propene. By employing the classical ensemble method, we showed electron–electron correlations in the calculated momentum distributions and recollision of the first ionized electron with the ion core in the energy trajectories after DI in linearly polarized laser fields. In order to reveal the DI dynamics of cyclopropane and propene molecule, we take into account all the recollision trajectories, which shows that both recollision impact ionization (RII) and recollision excitation with subsequent ionization (RESI) mechanisms may be contributed to the molecular strong-field NSDI, because we can see the direct recollision trajectories, multi-recollision trajectories, and laser-induced trajectories. We hope this work would stimulate further investigations to reveal molecular structure effect and quantum features in the ionization process.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] |