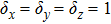† Corresponding author. E-mail:
Feed-forward gene transcriptional regulatory networks, as a set of common signal motifs, are widely distributed in the biological systems. In this paper, the noise characteristics and propagation mechanism of various feed-forward gene transcriptional regulatory loops are investigated, including (i) coherent feed-forward loops with AND-gate, (ii) coherent feed-forward loops with OR-gate logic, and (iii) incoherent feed-forward loops with AND-gate logic. By introducing logarithmic gain coefficient and using linear noise approximation, the theoretical formulas of noise decomposition are derived and the theoretical results are verified by Gillespie simulation. From the theoretical and numerical results of noise decomposition algorithm, three general characteristics about noise transmission in these different kinds of feed-forward loops are observed. i) The two-step noise propagation of upstream factor is negative in the incoherent feed-forward loops with AND-gate logic, that is, upstream factor can indirectly suppress the noise of downstream factors. ii) The one-step propagation noise of upstream factor is non-monotonic in the coherent feed-forward loops with OR-gate logic. iii) When the branch of the feed-forward loop is negatively controlled, the total noise of the downstream factor monotonically increases for each of all feed-forward loops. These findings are robust to variations of model parameters. These observations reveal the universal rules of noise propagation in the feed-forward loops, and may contribute to our understanding of design principle of gene circuits.
Genes are the carriers of genetic information, and the expression of genes is the basic process of life system. Many genes and proteins are involved in the life process and they constitute rather complicated regulatory networks. Although regulatory networks are very complex, recent research has shown that these complex gene regulatory networks can be decomposed into many typical small network motifs, such as auto-regulation motifs, feedback motifs, feed-forward loops, etc.[1]
Random fluctuations in gene transcriptional regulatory networks are unavoidable, as whether chemical reactions occur is probabilistic and the numbers of many genes, RNAs and proteins are all small in each cell.[2] Noise and fluctuations have permeated into all levels of the biological system, from the basic molecular, sub-cellular processes to the kinetics of tissues, organs, organisms and populations.[3] There is a large amount of random noise in DNA replication, transcription regulation and protein translation in organisms. It is very well known that the internal noise and the external noise are both inevitable in a biochemical reaction system.[4–6] The internal noise that comes from stochastic fluctuations of random chemical reaction events[7–10] in biochemical systems can result in gene mutation and evolution,[11] cell-to-cell variability,[7,12,13] and even different observable phenotypes.[11]
A large number of motifs exist in the gene transcription regulatory networks, i.e., auto-regulation motif, feedback motif, feed-forward loop. We can find that the number of units that make up these simple modules is relatively few, so the noise should not be ignored. It is recognized that feedback loops and noise play important roles in a wide variety of biological processes, such as galactose regulation,[14] calcium signalling,[15,16] p53 regulation,[17] cell cycle,[18,19] and cell fate decision in budding yeast.[20,21] Some studies indicated that positive feedbacks tended to magnify noise and negative feedbacks typically attenuated noise.[22–24] However, most of the positive feedback loops are bistable where stationary noise in a real system is rarely observed, and only transition rate can be measured. The difficulty in predicting the transition rate lies in the fact that noise and transient kinetics (e.g. due to step-response) have often similar effects on transition rate and it is difficult to distinguish between them. Some researchers have pointed out that the gene expression noise is a key factor in determining the rate of transition in the bistable range.[25] Nevertheless, other studies have shown that the positive feedbacks can attenuate noises and strong correlation does not exist between the feedback signals (negative or positive) and the noise attenuation characteristic.[26,27]
As typical of biological motif, the feed-forward loop consists of two input transcription factors and a target gene. One of the transcription factors regulates the other, conjointly regulating the target gene. Each of the transcription interactions among three genes in the feed-forward loop can be either activation (positive) or inhibition (negative), therefore the feed-forward loop has eight possible structural types with different activation and repression interactions. According to the different regulatory functions between direct regulation branch and indirect control main path, four of them are considered as coherent feed-forward loops (CFFL), the other four structures are called incoherent feed-forward loops (IFFL). The effects of transcription factors are integrated in the promoter region of target gene. The expression of target gene is modulated on the basis of the concentration of transcription factors bound to their inducers. This modulation is described by the cis-regulatory input function, including AND-gate logic and OR-gate logic. In the past decades, more and more attention has been paid to the functions, structures, as well as noise characteristics of feed-forward loops.[22,28–42] The regulation mechanisms of feed-forward loops in cell fate decisions in budding yeast were studied.[20] It is found that the coherent feed-forward loop responds only to persistent stimuli and rejects transient input pulses as shown in Ref. [28]. The noise characteristics of the feed-forward loop in the steady state have been studied by using the Langevin formalism and numerical simulation.[32] Furthermore, the principle of noise decomposition and the mechanism of noise transmission in a coherent feed-forward transcriptional regulatory loop has been studied in our recent work.[43]
However, in our research above, only the kind of coherent feed-forward loop with OR-gate logic was considered, but the AND-gate logic was not taken into account. In addition, the incoherent feed-forward loops with AND-gate logic were not explored either. In order to obtain more general observations about the fluctuation decomposition and noise propagation in the feed-forward transcriptional regulatory loop, we will investigate the stochastic dynamic behaviors of these various types of feed-forward transcriptional regulatory loops. The rest of this paper is organized as follows. In Section
A feed-forward loop is composed of three components: two transcription factors X and Y, where the former regulates the latter, and a target gene Z, where X and Y both bind the regulatory region of Z and jointly modulate the transcription rate (Fig.
For coherent feed-forward loops, the efficacy of the transcription factors X and Y is integrated in the promoter region of target gene Z, thus the expression level of Z is regulated according to the transcriptional concentrations of X and Y bound to their inducers. This modulation is represented by the cis-regulatory input function.[44–46] Cis-regulatory input functions contain (i) AND-gate logic, in which both X and Y are needed in order to express target gene Z, (ii) OR-gate logic, in which either X or Y is adequate to express downstream gene Z.
Therefore, the feed-forward loop consists of twelve different types as shown in Fig.
In order to study the general noise characteristics of twelve different types of feed-forward loops, the theoretical formulas of noise in various kinds of feed-forward loops are derived by using the linear noise approximation, numerical results by Gillespie simulation,[47] and their results are compared with the analytic results.
In accordance with the biochemical reaction rules,[28] a set of ordinary differential equations is established for the mathematical modelling of relevant gene regulatory networks.[28] The process of generation and annihilation of upstream factor X is considered, the feed-forward loop model has three variables. Take a kind of coherent feed-forward loop with AND-gate logic (Fig.
 |
 |
 |
No matter what initial states the system starts from, it eventually tends to be a steady state, that is, the attractor of the system. The feed-forward loop has an attractor as the attracting fixed point
 |
In the case of a small number of molecules, the random noise can be described accurately by the master equation. A theoretical deduction about the noise formula is given below, proceeding from the master equations. The numbers of the expression productions are expressed as Ni, 





In the case that the Hill coefficient n = 1, the Hill constants 
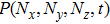
 |



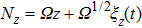




 |
Consequently,
 |
 |
The linear noise approximation is summarized by[50,51]
 |

 |
 |
 |
 |



To measure how the balance between production and elimination of Ni is affected by Nk,[2,52–56] the logarithmic gain is defined as 



 |
Under the steady state (i.e., 


Therefore, the drift matrix A is rewritten as 
 |
 |
 |
 |
 |




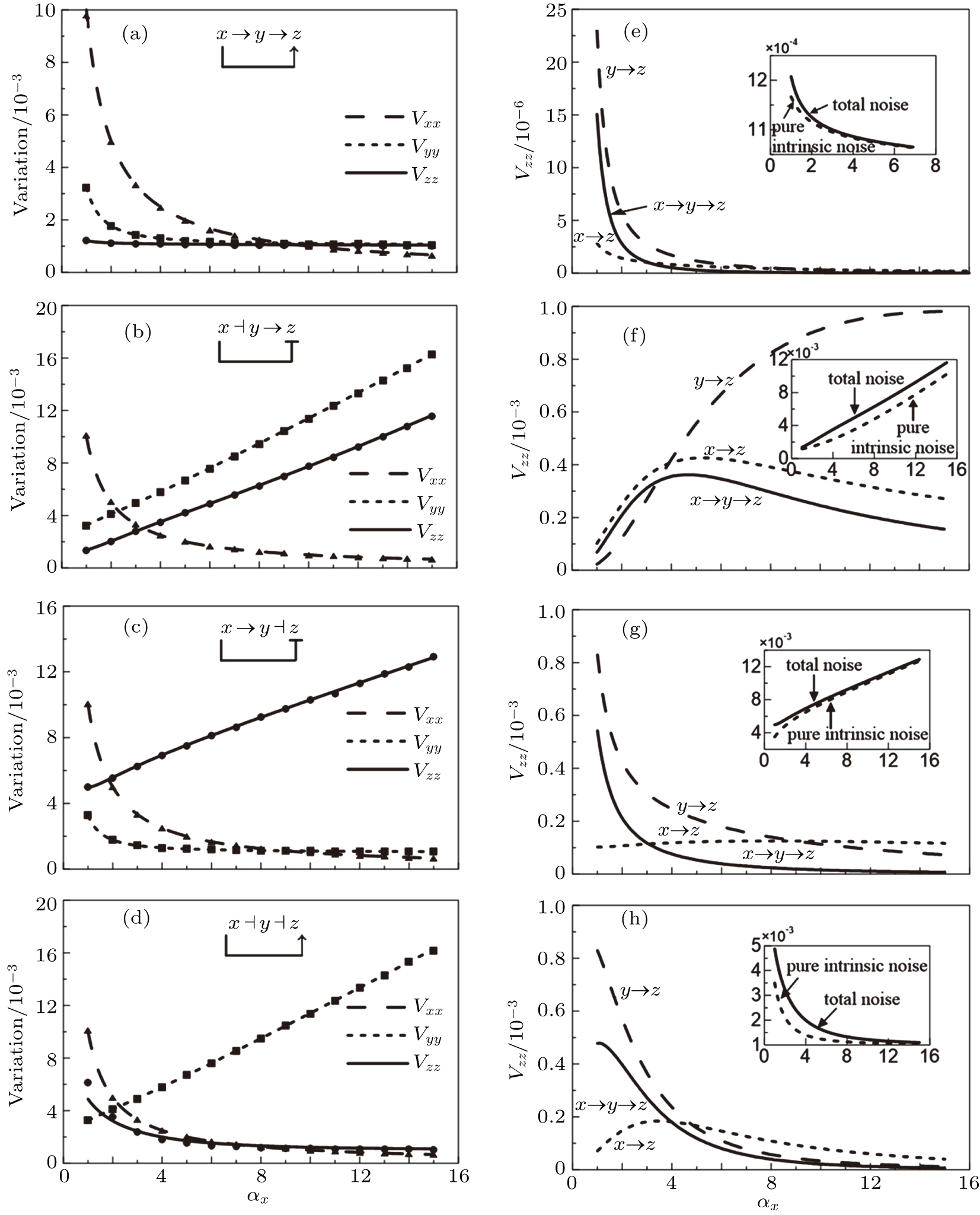
The method we proposed in the present article allows us to calculate the contributions of different components to the total variability of a system output. According to the theoretical formulas of the variance and the normalized variance obtained by the above calculations, we can make a more profound analysis about how the noise propagates in the feed-forward loop. Some interesting results for the twelve kinds of the feed-forward loops are found by the noise decomposition method (Fig.
In a biological system, incoherent feed-forward loops are also as common as coherent feed-forward loops. Hence we study noise characteristics of different nodes in these small gene networks as observed in Eqs. (
As illustrated in Figs. 

As shown in Figs. 
As demonstrated in Eq. (
This novel result indicates that the noise control function for downstream regulation not only is related to the positive or negative regulation property, but also is associated with the regulation step-number in the pathway. Even though the regulation sign is positive, mediated by intermediate factors, upstream factors can still implement the noise suppression control, hence indirectly reducing the randomness of downstream factors.
The noise characteristics and noise decomposition of coherent feed-forward loops with OR-gate logic have already been investigated in our recent work.[43] Several other types of coherent feed-forward loops with OR-gate logic are further considered here. According to the theoretical Eqs. (
As demonstrated in Fig.
As observed from the noise decomposition diagram of downstream factor Z, i.e., Figs. 
From Eq. (
According to the theoretical Eqs. (
Interestingly, the total noise level of Z exhibits a monotonic upward trend for the feed-forward loop when the regulation property of branch road is negative as exhibited in Figs.
In order to further explore how the noise propagates in the feed-forward loop, the noise decomposition results of downstream factor Z are described in Figs.
The recent development in fluorescent microscopy technology allows the proteins such as green fluorescent protein (GFP) to be measured in vivo in individual cells. The time-lapse microscopy was used to measure noise in the expression of GFP in individual cells. Intrinsic noise is due to the stochastic nature of the birth and death processes involved in reactions while extrinsic noise arises from the cell-to-cell variation of kinetic parameters. The current experimental technology can already be used to measure the different contributions of intrinsic and extrinsic noise to the total variability of a molecular system. Our method has also decomposed the noise in a biochemical system into contributions from different individual molecules. Hence, it is possible to design some gene circuits to test our theoretical findings. The different contribution is particularly important as it may indicate potentially new therapeutic targets. Always, it is required to search appropriate target molecules in order to switch off or turn on a certain pathway. However, the interventions for different components in the upstream have different functions. The precise knowledge about the dynamics of signal transduction and noise propagation may help us find the new potential therapeutic targets. Quite generally, the ability to dissect noise propagation through biological systems does enable researchers better to understand the role of noise in function (and evolution), and will also enable synthetic biologists to either harness or dampen the effects of noise in molecular signaling and response networks.
In our work, we do not study the relationship between expression level and noise, while the dependence of stochastic noise on αx is explored. Fortunately, we also find that the noise curve for each kind of feed-forward loop has its particular feature. Besides the fact that the dependence of total noise on αx provides rich information about the structures of feed-forward loops, the noise components in total noise, including first-step and two-step propagation noises, show some further features. That is to say, for different structures of feed-forward loops, the differences illustrated from the dependence of noise components on αx are clearer than those from the dependence of total noise. With this information about noise propagation, we may infer the network structure in higher precision.
The transmission mechanisms of complex signals in biological systems have been investigated widely.[59,60] It is well known that noise is unavoidable for these cellular systems.[61–63] Therefore, more and more studies focused on the mechanism of noise propagation in a biochemical network.[64,65] In this paper, the noise characteristics and propagation mechanisms of twelve kinds of feed-forward gene transcriptional regulatory loops are investigated. The theoretical formulas of total noise and noise decomposition are theoretically derived by using linear noise approximation. Our approach is widely applicable and does allow us to disentangle the different contributions to system output resulting from the different components in a system. Three general results of noise transmission in these feed-forward loops are found. (i) For incoherent feed-forward loops with AND-gate logic, the two-step noise propagation of upstream factor is negative. (ii) For coherent feed-forward loops with OR-gate logic, the one-step propagation noise of upstream factor is non-monotonic. (iii) For all feed-forward loops, if the branch road of the feed-forward loop is negatively controlled, the total noise of downstream factor monotonically increases. The potential mechanisms and biological relevances are presented.
Due to lack of experimental data, it is difficult to build a quantitative feed-forward loop model. Only a coarse model of feed-forward loop is used to explore the qualitative behaviors. In order to check the robustness of our observations, the theoretical results are further investigated in a much wider range of parameters.
First, for incoherent feed-forward loops with AND-gate logic, we study the two-step propagation noise in order to verify whether upstream factors can inhibit signal noise indirectly by reducing the randomness of downstream factors. As an example, a kind of incoherent feed-forward transcriptional regulatory network (Fig.
Second, for coherent feed-forward loops with OR-gate logic, it is found that the one-step propagation is non-monotonic. When the control parameter is at a moderate value, the noise intensity of downstream factor is magnified. In contrast, the further reduction or enlargement of control parameters, the randomness of the downstream factors can be optimized under the first-order propagation. In view of coherent feed-forward gene regulation network with OR-gate logic (Fig.
Third, for all kinds of feed-forward loops, as the concentration of upstream factor increases, the concentration of downstream factors will be suppressed if the regulatory branch is negatively regulated. According to the inverse relation of the square root of noise to molecular number, it is obvious that the noise of downstream factor is enhanced with upstream component increasing.
Fourth, it should be pointed out that the gap between the total noise and internal noise seems small in these results, however under different parameter values, it is noticed that the total noise may not be dominated by the internal noise. In particular, when the production rate and degradation rate of Z are adjusted, the propagation noise becomes large, close to the internal noise.
It should be pointed out that we discuss the noise propagation varying only the expression level of the upstream factor αx. However, the effect of other factors can be studied in a similar way. In particular, we can give some qualitative predictions if δx and αx are fixed while another reaction rate is selected as the control parameter. (i) When treating αy as the parameter, for incoherent feed-forward loop of AND-gates, the steady state value of X is a constant and the normalized variation Vxx is unchanged. Moreover, based on Table 
The noise decomposition algorithm allows us to investigate in detail how the noise propagates through a reaction system and affects the cellular process. What can a biologist learn from our results in gene circuit design? A simple discussion for the biological meaning of the noise propagation in feed-forward loop is provided below.
The structure of the control network determines the function and dynamics of the network. While the topological properties of the control network are always unknown, it is necessary to predict the network structure from the dynamical behaviors of biological network. An especially interesting topic is how to deduce the corresponding network from the underlying stochastic dynamics. For example, the authors have shown that for a genetic system with a single transcription factor (an activator can act as an inhibitor when bound to sites downstream of the TATA box), the precise amount by which noise changes with expression is specific to the regulatory mechanism of transcription and translation that acts on each gene.[66] Moreover, for feed-forward loops with two transcription factors, the previous theoretical and experimental research also has shown that the different feed-forward loops exhibit different relationships between mean expression and noise.[67] Our work provides a systematic study about how the noise is distributed and controlled in the feed-forward gene transcriptional regulatory loops. The theoretical method of noise propagation through these three-node, feed-forward loops may allow researchers to understand the role of noise on cellular signal transduction in more complex biological networks. If we can design genetic circuits and measure these noise components, perhaps the network structure can be inferred with higher precision.
We believe that these results will motivate the further investigation on the transmission of biological noise and potential function on evolution. Obviously, it is a challenge to verify these theoretical observations with the real biological systems or experiments. However, with the development of yeast genetics, time-lapse fluorescent microscopy, single cell assays, and microfluidic devices, it is possible to trace the dynamics of key proteins in some simple synthetic gene circuits. Therefore, the stochastic noise and propagation mechanism could be tested by this single cell technology.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] | |
| [41] | |
| [42] | |
| [43] | |
| [44] | |
| [45] | |
| [46] | |
| [47] | |
| [48] | |
| [49] | |
| [50] | |
| [51] | |
| [52] | |
| [53] | |
| [54] | |
| [55] | |
| [56] | |
| [57] | |
| [58] | |
| [59] | |
| [60] | |
| [61] | |
| [62] | |
| [63] | |
| [64] | |
| [65] | |
| [66] | |
| [67] |