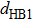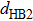|
Qin Jing-Yu1, 2, Geng Yi-Zhao3, 4, Lü Gang5, Ji Qing3, 4, 6, †, Fang Hai-Ping1, ‡
|
|
(color online) Docking process of β10. (a) Distance–time curves of
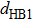 ,
,
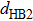 ,
,
 , and
, and
 in the β10 docking process with four steps. Three black arrows indicate the positions of three typical conformations shown in panels (b), (c) and (d). (b) Conformation of unbound β10 at ∼1.5 ns (first arrow) in the first step. The distances are all in unit [Å]. (c) Conformation of β10 in the metastable state at ∼4.8 ns (second arrow) in the second step. Two water bridges are formed between NL and the motor domain, which make a key contribution to the formation of the metastable state. (d) Conformation of β10 in the intermediate state between the second and third step at ∼6.7 ns (third arrow). Two water bridges are formed with the second one formed between the sidechain of Asn334 and the acceptor site of HB4 on Gly77 rather than between the donor and acceptor site of HB4 as in the case of panel (c).
in the β10 docking process with four steps. Three black arrows indicate the positions of three typical conformations shown in panels (b), (c) and (d). (b) Conformation of unbound β10 at ∼1.5 ns (first arrow) in the first step. The distances are all in unit [Å]. (c) Conformation of β10 in the metastable state at ∼4.8 ns (second arrow) in the second step. Two water bridges are formed between NL and the motor domain, which make a key contribution to the formation of the metastable state. (d) Conformation of β10 in the intermediate state between the second and third step at ∼6.7 ns (third arrow). Two water bridges are formed with the second one formed between the sidechain of Asn334 and the acceptor site of HB4 on Gly77 rather than between the donor and acceptor site of HB4 as in the case of panel (c).
|