† Corresponding author. E-mail:
Thermodynamic properties of Cd0.25Zn0.75Se alloy are studied using quasi harmonic model for pressure range of 0 GPa−10 GPa and temperature range 0 K−1000 K. The structural optimization is obtained by self-consistent field calculations and full-potential linearized muffin-tin orbital method with GGA+U as an exchange correlation functional where U = 2.3427 eV is Hubbard potential. The effects of temperature and pressure on bulk modulus, Helmholtz free energy, internal energy, entropy, Debye temperature, Grüneisen parameter, thermal expansion coefficient, and heat capacities of the material are observed and discussed. The bulk modulus, Helmholtz free energy, and Debye temperature are found to be decreased on increasing temperature while there is an increasing behavior with rise of the pressure. Whereas the internal energy has increasing trend with the rise in temperature and it almost remains insensitive to pressure. The entropy of the system increases (decreases) with rise of pressure (temperature).
Wide band gap semiconductors are very important due to their large number of applications in optoelectronic devices like light emitting diode, optical wave guide, solar cells, solid state lasers, and photodetectors. The ZnSe is one of them which crystallizes in cubic zinc blend structure at ambient pressure. It has a distinct property of reversible transformation so it is being used in optical memory devices.[1] Cd-dopped ZnSe ternary alloys are one of the II–VI semiconductor materials. They have high stability along with wide band gap[2] and they are used for fabrication of photoluminescent, electroluminescent, photovoltaic, and photoconductive devices.[3–6] Experimentally CdZnSe thin films have been prepared by vacuum evaporation,[7] molecular beam epitaxy (MBE),[8] electron beam pumping,[9] electrodeposition,[10] and chemical bath deposition (CBD).[6] The experimental studies on CdZnSe have been done for structural properties,[11,12] optical properties,[6,11,13–17] dielectric properties,[15] and magnetic properties.[18]
The density functional theory (DFT) study on CdZnSe includes structural properties[19,20] to measure lattice constants and bulk modulii; elastic properties[21] to measure elastic stiffness constants; electronic properties[19,20] to measure band gap energies; and optical properties[20] to measure dielectric functions, refractive index, and extinction coefficient. But to our knowledge, there is no work found up to now about thermodynamical properties of CdZnSe. In the present study we fill this gap and study the thermodynamical properties of highly correlated ternary alloy Cd0.25Zn0.75Se with pressure range of 0 GPa–10 GPa and temperature range of 0 K–1000 K. The Cd0.25Zn0.75Se has direct band gap of 1.90 eV[22] which corresponds to 652.6-nm wavelength. This material is useful in fabrication of light emitting diode capable to emit red light. The rest of the portion of visible spectrum may be explored with other ratio of composition of Cd and Zn in ternary alloy. In the highely corelated systems, the interaction of electrons with phonons are important which can give rise lattice distortions.[23] As a result phonon frequencies may change and consequently thermodynamic properties will be affected. The thermodynamic properties of highly correlated systems should be calculated by using Coulomb repulsion parameter U,[24] called Hubbard potential. The Hubbard potential is optimized by the method proposed by Gunnarsson et al.[25] In this method, we used 2 × 2 × 2 supercell and hopping integral of d-orbital of central zinc atom is set to be zero. When atoms are considered to be embedded in a polarizable surroundings then the parameter U is the energy required to move an electron from one atom to another. So in this case U is equal to the difference of ionization potential (Ei) and electron affinity (Ea). Removing an electron from a site will polarize its surroundings, thereby lowering the ground state energy of the (N − 1) electron system.[26] Thus Ei = EN−1 − EN and Ea = EN − EN+1, and the optimized value of the Hubbard potential is then U = (EN−1 − EN) −( EN − EN+1), where EN±1 are the ground state energies of ( N±1)-electron system. The optimized value is used to calculate structural optimization of Cd0.25Zn0.75Se in WIEN2k code. The structural optimization data are then used to find thermodynamic properties of the ternary alloy.
This paper is organized as follows. Section
The optimized calculations are performed by using DFT implemented in WIEN2k code[27] with full potential linearized augmented plane wave (FP-LAPW+lo) method with local orbital. The generalized gradient approximation with Hubbard potential (GGA+U) is used as exchange–correlation functional where U = 2.3427 eV parameter is optimized by the method discussed in Section
The thermodynamic properties are determined by using quasi-harmonic Debye model which is implemented in Gibbs code.[28] The energy volume optimization data are used as input to determine the pressure and temperature dependence of Helmholtz free energy, internal energy, entropy, Debye temperature, and heat capacity. In the quasi-harmonic Debye model the non-equilibrium Gibbs function G*(V;P,T) can be written as[29]
The equilibrium geometry is achieved by minimizing the Gibbs function with respect to volume of the unit cell at constant pressure and temperature, i.e.
By using minimizing condition in Eq. (

The bulk modulus is a numerical constant used to describe the elastic properties of a solid under pressure. It is a measure of ability of a substance to withstand the changes in volume under compression. It depends on pressure and temperature as shown in Fig.
The Helmholtz free energy is an important parameter to determine the stability of a structure. A structure with more negative value of the Helmholtz free energy will be considered to be more stable. The Helmholtz free energy at any temperature T can be written easily in the scope of standard thermodynamics as
The Helmholtz energy is decreased and the internal energy is increased with the increase of temperature and almost the internal energy is found to be insensitive to pressure above 200 K where the Helmholtz energy is increased on increasing pressure from 0 GPa–10 GPa. At low temperature limit, Fvib weakly depends upon pressure while at high temperature limit, it strongly depends on pressure whereas Uvib is found to be more sensitive to pressure at low temperature. At low temperature limit, the atomic oscillators have small amplitude of oscillations and hence an increase in pressure induces a prominent effect on the internal energy and insensitive to Helmholtz energy of the system. While at high temperature limit, pressure has negligible effect on the internal energy and prominent effect on the Helmholtz energy of the system because harmonicity is now converted into anharmonicity. The internal energy at 0 K is attributed to the existence of zero point motion and the calculated Fvib and Uvib at absolute zero is 12.01314 kJ/mol. The increase in Helmholtz energy on increasing pressure is attributed to decrease in entropy at certain temperatures. The effect of hydrostatic pressure and temperature on entropy of Cd0.25Zn0.75Se is shown in Fig.
The Debye temperature is a key quantity in the quasi-harmonic Debye model which is related to many properties like elastic constants, thermal expansion, melting temperature, and specific heat. The Debye temperature at zero kelvin and zero Pascal is 428.10 K as shown in Fig.
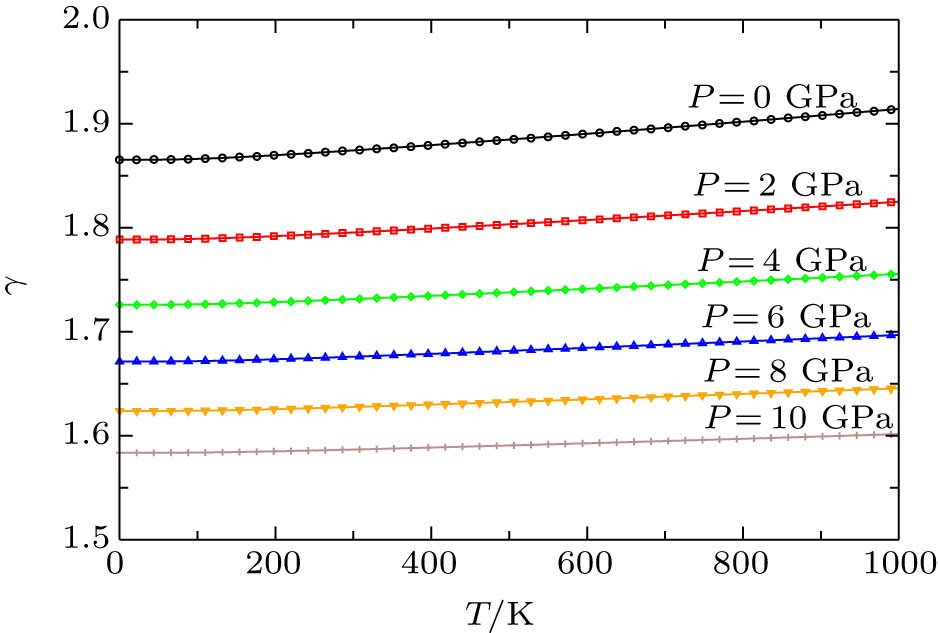
The Grüneisen parameter reflects the anharmonicity in the crystal that how much phonon vibrations are deviating from harmonic oscillations. Figures
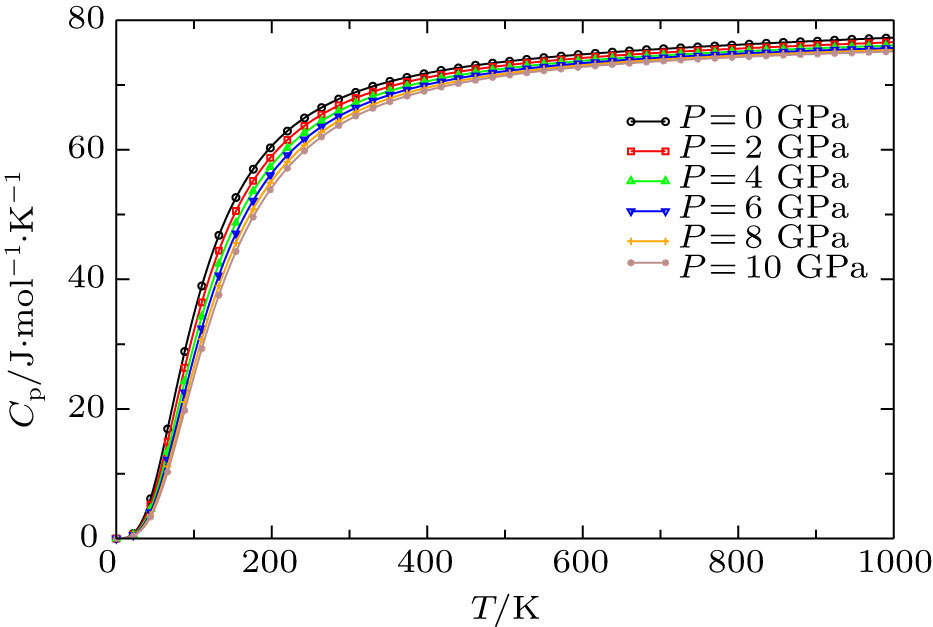
The heat capacity at constant volume Cv and at constant pressure Cp on basis of Debye quasi-harmonic approximation as a function of temperature at different pressures is shown in Fig.
Figure
 | Fig. 10. (color online) The pressure dependence of Helmholtz energy, internal energy, and entropy at T = 300 K. |
The effect of pressure on Debye temperature and heat capacity at T = 300 K is shown in Fig.
The pressure and temperature dependences of thermodynamic properties of Cd0.25Zn0.75Se have been calculated by using FP-LAPW+lo method in the framework of DFT and Debye quasi-harmonic approximation which are implemented in WIEN2k and Gibbs codes respectively. The Helmholtz free energy, entropy, and thermal expansion coefficient are found to be more sensitive to pressure in high temperature limits while the internal energy is pressure-sensitive in low temperature limits. The specific heat at constant volume approaches to classical limit at T = 800 K. The Grüneisen parameter and thermal expansion coefficient decrease with the rise of pressure. This means that anharmonicity in our new material decreases and this material starts to expand at low temperature by increasing pressure on it.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] |