† Corresponding author. E-mail:
Project supported by the Program of International Science and Technology Cooperation of China (Grant No. 2014DFG60230), the National Basic Research Program of China (Grant No. 2010CB934504), the Strategically Leading Program of the Chinese Academy of Sciences (Grant No. XDA02040100), the Shanghai Municipal Science and Technology Commission, China (Grant No. 13ZR1448000), and the National Natural Science Foundation of China (Grant Nos. 91326105 and 21306220).
We have investigated the expansion and bursting of a helium nano-bubble near the surface of a nickel matrix using a molecular dynamics simulation. The helium atoms erupt from the bubble in an instantaneous and volcano-like process, which leads to surface deformation consisting of cavity formation on the surface, along with modification and atomic rearrangement at the periphery of the cavity. During the kinetic releasing process, the channel may undergo the “open” and “close” states more than once due to the variation of the stress inside the nano-bubble. The ratio between the number of helium atoms and one of vacancies can directly reflect the releasing rate under different temperatures and crystallographic orientation conditions, respectively. Moreover, a special relationship between the stress and He-to-vacancy ratio is also determined. This model is tested to compare with the experimental result from Hastelloy N alloys implanted by helium ions and satisfactory agreement is obtained.
The formation of helium bubbles leads to high-temperature intergranular embrittlement, which eventually leads to the degradation of the mechanical integrity of materials,[1,2] and then affects the lifetime and the safety of the reactor at some level. The Hastelloy N alloys as the structural material in the molten salt reactor (MSR) contain 68% 58Ni (nickel, Ni) and 26% 60Ni, both of which can generate helium (He) by the (n, α) transmutation reaction, as follows: 58Ni + nf → 55Fe + 4He and 60Ni + nf → 57Fe + 4He. An estimated 40 ppm of helium was generated in molten salt breeder reactor (MSBR) over 30 years.[3] Liu et al.[4] described the surface blistering and cracking phenomenon experimentally on Hastelloy N alloys implanted with 30-keV helium ions and doses of 1 × 1015, 5 × 1015, and 1 × 1016 He/cm2 respectively, and the temperature is 500 °C. The mean range of the implanted helium was 240 nm and the irradiation area is around 200 nm, while for the peak value the irradiation is about 100 nm. The study suggested that the observation of surface blistering and the nano-bubble sizes phenomenon are within the range from 0.5 to 3.0 μm. The distribution and size of nano-bubble are not uniform, rather than alignment or aggregation. Of interest here were the results which showed in the implanted area the single nano-bubble is mainly sphere and even the outline of the capped nano-bubble forms as shown in Ref. [4], and the nano-bubble breakups and the cracks appear on the surface randomly as the dose increases.
The formation of gas-filled blisters in the surface region of irradiated solids has been also observed.[5–7] The blisters can rupture in energetically favored regions and thereby release bursts of gas. Blistering plays a very important role in issue about helium embrittlement, since it can lead to (i) serious damage and erosion of bombarded surfaces and (ii) the release of gases which will contaminate the secondary circuit. Much experimental research about the behavior of He nano-bubble in metals has been carried in recent decades.[8–12] For instance, Cipiti et al.[12] implanted helium and deuterium into polished tungsten specimens under high temperature. They analyzed the effects of the energy of the incident ion beam and temperature on the surface structures. Zenobia et al.[13] studied that the retention and surface pore formation with 30 keV 3He implanted into tungsten at temperatures ranging from 850–1000 °C. The results indicated that the threshold for the surface pore formation occurs between ~ 5 × 1016–4 × 1017 He+/cm2 and with the higher implanted influence, both surface and sub-surface pore formation is also observed to increase. Generally, the gas driven model and the lateral stress driven model are used to explain the mechanism of blistering on the surface. For example, Sefta et al.[14,15] investigated the bursting behavior of sub-surface, helium bubbles pressure evolution and the resulting tungsten surface morphology. Their results provided insight into the conditions and mechanisms leading to various tungsten topology changes and surface roughening occurs as single adatoms migrate to the surface, prismatic loops glide to the surface to form islands, and ultimately as over-pressurized helium bubbles burst. Zhang et al.[16] studied helium bubble formation and releasing near the titanium surface. Their results suggest that the helium bubble burst results in pores in the metal surface and the size of the resultant surface pore depends on the initial bubble diameter. Ohno et al.[17] have investigated that the influence of crystallographic orientation on the formation of helium bubble and nanostructure by using ITER grade tungsten (W) exposed to helium plasma. The helium bubbles with a large helium pressure move the W lattice along the slip face. El-Atwani et al.[18] observed that the largest bubbles were formed on the grain boundaries, and future work will focus on the bubble coalescence and the effect of grain orientation. Though there has been much research about the helium bubble releasing near the metal surface, there are still problems left behind, such as, how the orientation affects the helium nano-bubble releasing and how the environment temperature and He-to-vacancy ratios affect the releasing process of helium bubble from the metal surface and even the stress variants in the complete releasing process.
In the present work, we investigated the evolution of helium nanobubbles near the nickel surface in terms of the variation of orientations, helium-to-vacancy ratios, temperatures and release mechanisms involved at the atomic level to provide a reasonable picture of the blistering formation and gas release results reported by Liu et al.[4]
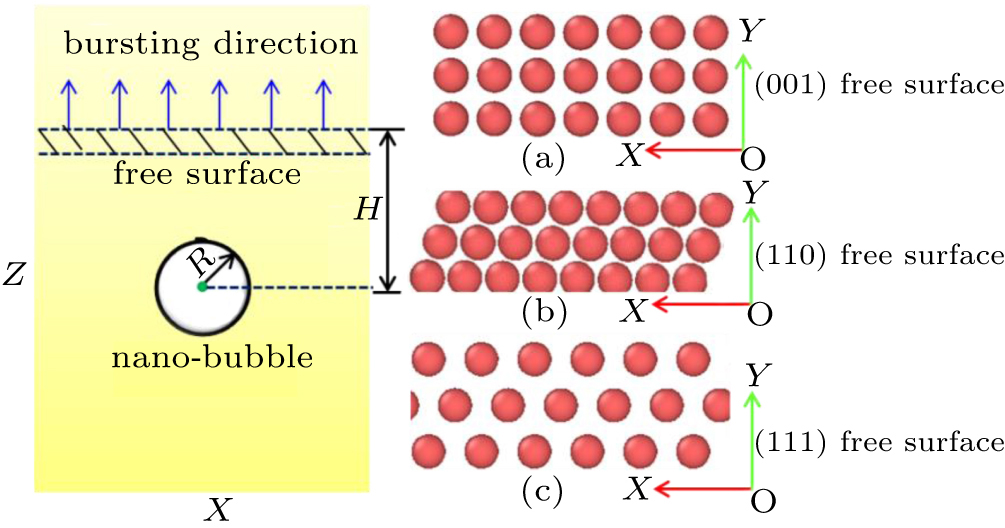
All calculations were performed using the molecular dynamics (MD) simulation with the atomic/molecular massively parallel simulator (LAMMPS) software package.[19] The atomic interactions of Ni–Ni, Ni–He, and He–He atoms were described by the modified analytic EAM model (MAEAM),[20–29] the Morse potential,[30] and the Lennard–Jones potential,[22] respectively. More information about the formulas and parameters can be found in Ref. [32]. Periodic boundary conditions were applied in the X- and Y-directions, while the Z-direction was a free surface. The [001], [1 − 10], and [111] single crystal surfaces were considered to be the helium nano-bubble releasing paths, respectively. To present the atoms moving in the vertical direction due to the escape of helium atoms, the bottom three layers of the simulation box were frozen in the simulation process.
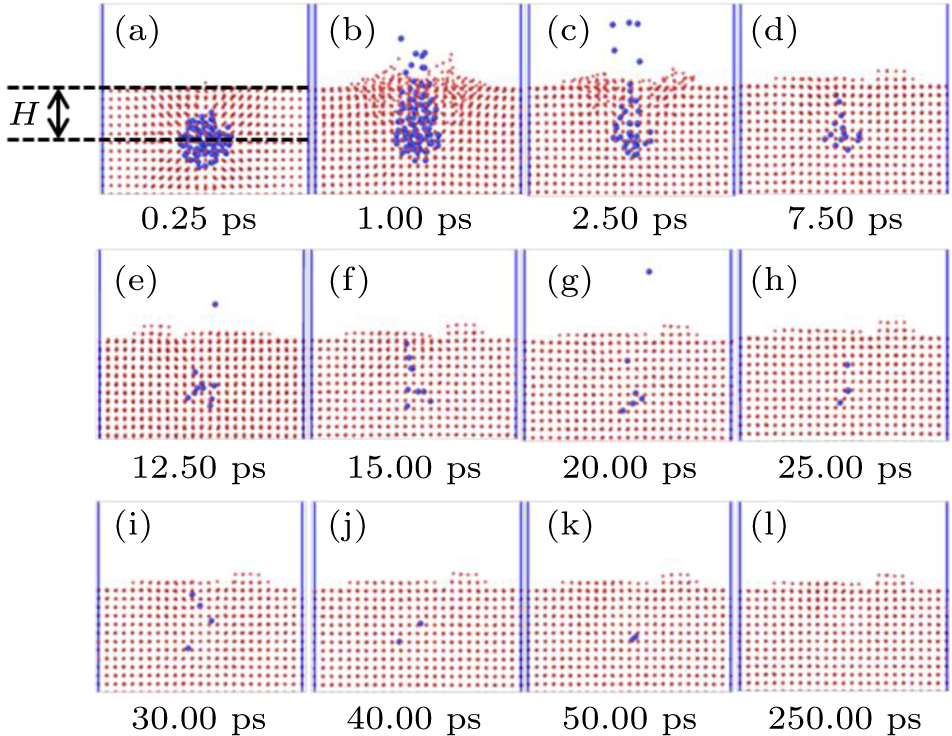
Three-dimensional periodic computational cell of 12a0 × 12a0 × 12a0 was used to eliminate surface effects, where a0 is the lattice constant of face-center cubic nickel crystal (a0 = 3.5157 Å). The temperature was set to 300 K, 500 K, 800 K, and 1000 K, respectively. The Nosé–Hoover style non-Hamitonian equation of motion describes the constant temperature technique[33] with a time step of 0.5 femtosecond (fs). The helium-to-vacancy ratios were 1:0, 2:1, 3:1, and 4:1, respectively. The Polak–Ribiere version of the conjugate gradient (CG) algorithm was used to relax the whole simulation systems.[33] There was no pressure control in the whole simulation process. The total simulation time was 600.0 picosecond (ps). The equilibrium helium nano-bubble was created by moving a certain nickel volume to initially form a spherical void with a radius of 8.0 Angstrom (Å) and then inserting helium atoms for the specific ratio with vacancies into it at once. We only considered a spherical helium bubble with a radius of 7.0 Å to ensure the stability of helium nano-bubble for modelling. Furthermore, the depth (H) of the helium nano-bubble from the surface to the mass center of bubble will influence on the pressure of nano-bubble, and the behavior of nano-bubble releasing process. Here, we only considered the critical depth (H) which can exactly satisfy the pressure of nano-bubble releasing. In different initial conditions, such as temperature, helium-to-vacancy ratio, and single crystal free surface, the depth (H) needs to be adjusted to make the releasing process just appear. The sketch is shown in Fig.
Due to the limitation of experimental observation, it is very important to understand the evolution and release behavior of helium nano-bubbles near the crystalline surface and the corresponding mechanisms by simulation means. To illuminate the effect of crystallographic orientations on the behavior of the helium nano-bubbles, we simulated three crystallographic orientations, such as [001], [1 – 10], and [111], which are along the escaping direction of the helium nano-bubbles. For the three free surface orientations, the releasing process of helium nano-bubbles can be mainly divided into three stages: (I) the relaxing region of helium nano-bubbles below the free surface; (II) the escaping region of helium nano-bubbles due to over-pressurization inside them; (III) the stable region by the end of helium nano-bubbles releasing completely. Figure
The helium-to-vacancy ratios also have a great influence on the helium nano-bubble escaping near the surface. With the helium-to-vacancy ratio increasing, the inside pressure of the helium nano-bubble increases. As the pressure passes the limitation, the helium nano-bubble starts to escape toward the surface. Here, we only considered four helium-to-vacancy ratios, such as 1:0, 1:1, 2:1, and 3:1. Figure
The releasing process of the helium nano-bubble is a dynamics balance, which can reflect the channel formation connecting the helium nano-bubble to the near surface, then the helium atoms will easily escape from the matrix along this channel one by one or in the forms of smaller helium clusters until the new balance is built again. Thus, the formation of the channels and surface cavity is a great strategic point, which can accelerate the helium release from the near surface region.
Figure
Moreover, due to the options of different parameters, such as He-to-vacancy ratio, temperature and crystallographic orientations, etc., some helium atoms are detained, which is not shown here. In particular, the crystallographic orientations have great effect on the nano-bubble bursting. Parish et al.[36] have investigated that the normal-direction crystallographic orientation of the underlying grain controls the growth morphology by SEM observation. They pointed out that grain formed pyramids near-〈001〉|| normal direction (ND) formed wavy and stepped structures near-〈114〉 to 〈112〉|| ND, and remained smooth near-〈103〉|| ND. For the crystallographic orientations, such as Z[1 − 10] and Z[111], the surface morphology also transformed from the smooth free surface to the “pyramid” style or layer-by-layer or stepped style, or even the mixed style (both of them) after nano-bubble bursting and escaping from the surface, which was similar to the case in Z[001] crystallographic orientation. This suggested that the crystallographic orientation will directly influence the surface morphology during the nano-bubble bursting and escaping process.
Here, we consider the influence of crystallographic orientation and He-to-vacancy ratios at 500 K. The release rate also appears to have three stages: (I) the accelerating release rate from 0 to around 3.0 ps, which corresponds to the high pressure inside helium nano-bubbles; (II) the decelerating release rate from 3.0 ps to 10.0 ps, which may be associated with the decrease of the number of helium atoms, and (III) the constant process, relatively a low release rate, which is not shown here. For different crystallographic orientations, the maximum released rate is found along Z[001], then Z[1 − 10] and Z[111], as shown in Fig.
 | Fig. 5. (color online) Released rate of He atoms as a function of the simulation time at 500 K for different orientations with He-to-vacancy ratios: (a) 1:1; (b) 2:1; (c) 3:1. |
The different configurations of the surface layers with some critical running time are also shown in Fig.
Due to the helium atoms added into the system during the simulation only for one time, the bubble pressure is triggered to increase above equilibrium, which develops compressive strain in the surrounding nickel matrix. Under overpressure conditions within helium nano-bubbles, the bubble center of mass is relatively shallow compared to its size, the bubbles feel an attraction from free surface and tend to escape toward it along the direction of least resistance. With the bubble over-pressurization, pressure relief processes occur to relieve the internal pressure through prismatic interstitial loop-punching or by fracturing or deforming the surface ligament above the bubbles to the point of rupture, resulting in helium release. In some configurations, particularly when the bubbles are small and the ligament is thin. The pressure will simply increase until the ligament breaks without prior deformation of the nickel surface. In other cases, the bubble can increase the volume to reduce pressure. In the paper, we only consider the contribution of virial stress to the stress tensor for the whole simulation box. The macroscopic pressure P of a set of interacting atoms contained in a volume V can be derived in a number of methods, such as continuum mechanics, classical mechanics, and statistical mechanics.[38,39] All of these derivations result in the following well-established relationship:
Figure
 | Fig. 7. (color online) The stress variant as a function of the simulation time at 300 K for Z[111] orientation with He-to-vacancy ratios: (a) 1:0; (b) 1:1; (c) 2:1; (d) 3:1. |
The microscopic mechanism of helium release from a nickel near surface is investigated using MD method. A denuded zone has been observed in the modeling simulation, which can support the experimental observation. The channel usually needs to undergo “open” and “close” processes more than once during the releasing process. The present simulations provide a physical explanation for why the helium is released only from the near-surface region rather than through bulk diffusion. The helium release is an instantaneous process, with the helium erupting from the surface, creating surface deformation and the nucleation of a cavity on the surface. The cavity formation can further accelerate the helium release from the surface. This model is tested to compare with the experimental result from Hastelloy N alloys implanted by helium ions and satisfactory agreement is obtained. In addition, the study demonstrates that atomic-level simulations provide an important method to understand the dynamic process of helium release as well as determining the effects of the actual material microstructures on helium behavior in nuclear materials.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] |