† Corresponding author. E-mail:
In order to synthesize high-quality type-IIa large diamond, the selection of catalyst is very important, in addition to the nitrogen getter. In this paper, type-IIa large diamonds are grown under high pressure and high temperature (HPHT) by using the temperature gradient method (TGM), with adopting Ti/Cu as the nitrogen getter in Ni70Mn25Co5 (abbreviated as NiMnCo) or Fe55Ni29Co16 (abbreviated FeNiCo) catalyst. The values of nitrogen concentration (Nc) in both synthesized high-quality diamonds are less than 1 ppm, when Ti/Cu (1.6 wt%) is added in the FeNiCo or Ti/Cu (1.8 wt%) is added in the NiMnCo. The difference in solubility of nitrogen between both catalysts at HPHT is the basic reason for the different effect of Ti/Cu on eliminating nitrogen. The nitrogen-removal efficiency of Ti/Cu in the NiMnCo catalyst is less than in the FeNiCo catalyst. Additionally, a high-quality type-IIa large diamond size of 5.0 mm is obtained by reducing the growth rate and keeping the nitrogen concentration of the diamond to be less than 1 ppm, when Ti/Cu (1.6 wt%) is added in the FeNiCo catalyst.
Almost all natural diamonds have some defects. Especially type-Ib diamond contains a large quantity of nitrogen impurities which greatly influence the applications of its many excellent properties. Type-IIa large diamond (size> 1 mm) contains less nitrogen atoms (Nc < 1 ppm) and is free of impurities or inclusions.[1–6] Comparing with type-Ib large diamond, the mechanical and physical properties of type-IIa large diamond are greatly promoted, due to its high crystal quality and almost no crystal defects. These remarkable features enable it to give the maximum limiting function of type-IIa diamond. So, it can be used as infrared spectral window material, monochromator of x-ray synchrotron radiation, diamond anvil cells (DAC) of high pressure equipment, coal mine drill, etc.[7–12] Considering the scarcity of the natural resources and expensive price of perfect type-IIa diamond, study of the synthesis of type-IIa large diamond has practical significance.
In order to synthesize high-quality type-IIa large diamond, the selection of nitrogen getter and catalyst are very important. Nitrogen getter, which is free in the chamber, can react with nitrogen by generating more stable nitride, so it can avoid the nitrogen entering into the diamond.[4–17] The previous studies on type-IIa large diamond often focused on the nitrogen getter, especially on the commonly used nitrogen getter of Al and Ti/Cu.[6–14] Otherwise, catalyst refers to metal or alloy that is able to reduce the synthesis temperature and pressure conditions of diamond synthesis. The influences of different catalysts on Nc of synthetic type-Ib large diamond have been reported.[18–21] However, the influence of catalyst composition on eliminating nitrogen has not been reported. In order to synthesize type-IIa large diamond, we choose Ti/Cu as the nitrogen getter and added it to the typical NiMnCo catalyst or FeNiCo catalyst. Then, the different efficiencies of Ti/Cu on eliminating nitrogen with NiMnCo and FeNiCo catalyst are investigated in detail. High quality colorless type-IIa large diamond is synthesized by controlling the crystal growth rate, choosing a suitable catalyst, and determining the ratio of nitrogen getter related to catalyst.
The experiments on diamond crystal growth were carried out by TGM in a China-type cubic anvil high-pressure apparatus (CHPA) (SPD-6×1200). The assembly diagram of diamond synthesis is shown in Fig.
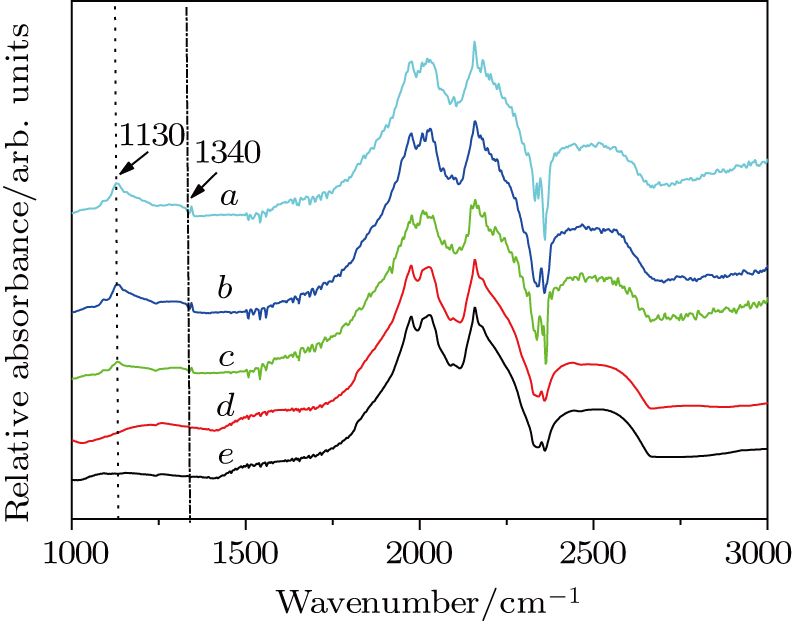
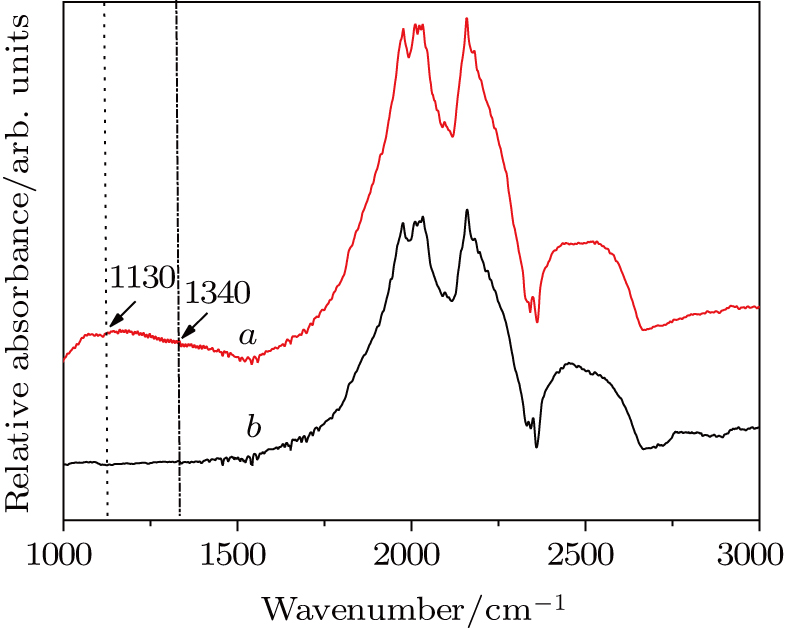
In the experiments, the pressure was estimated by the load of oil press, which was calibrated by a curve that was established on the pressure-induced phase transitions of elements of Bi, Ba, and Tl. The temperature was determined from the relationship between temperature and input power, which was calibrated using a Pt6%Rh-Pt30%Rh thermocouple.[15] The Nc in diamond was measured by the results of micro Fourier transform infrared (FTIR) spectroscopy. It was reported that Nc was proportional to the intensities of absorbance at 1130 cm−1 and 1344 cm−1 in an ideal spectrum, and determined from the equation 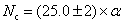
TGM under HPHT is that graphite first became diamond and dissolved in the catalyst, then, it began to diffuse to the seed under the driving of temperature gradient and then grew in the seed.[4–6] In one-dimensional approximation, the diamond crystal growth rate is proportional to the temperature gradient:
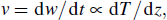 |
Fe-based alloy or Ni-based alloy is usually selected as the catalyst to synthesize large diamond under HPHT by using TGM. Then, type-IIa large diamonds are synthesized in the representative NiMnCo or FeNiCo catalyst and Ti/Cu is adopted as the nitrogen getter, because the efficiency of Ti/Cu on eliminating nitrogen is better than that of Al.[11,14] The Ti/Cu is different from Al which will generate easily the decomposition of AlN during diamond crystal growing.[4–6] So, Ti/Cu is chosen as the nitrogen getter to synthesize large diamonds. For nitrogen getter Ti/Cu, Ti reacts with N to eliminate nitrogen, and Cu dissolves TiC which is generated by Ti and C.[4–6]
Diamond syntheses are studied in NiMnCo alloy with the values of Ti/Cu ranging from 0.5 wt% to 2.0 wt%. Growth time and temperature of diamonds are 3 hour and 1280 °C, respectively. The temperature gradient is adjusted to 30 °C/mm and growth rate of diamonds was controlled to about 1.5 mg/h. The experimental results are shown in Table
| Table 1.
Results of diamond growth with different quantities of Ti/Cu in NiMnCo. . |
In order to determine the Nc content values in diamonds with different amounts of nitrogen getter in NiMnCo catalyst, all synthetic diamonds in Table
As shown in Table
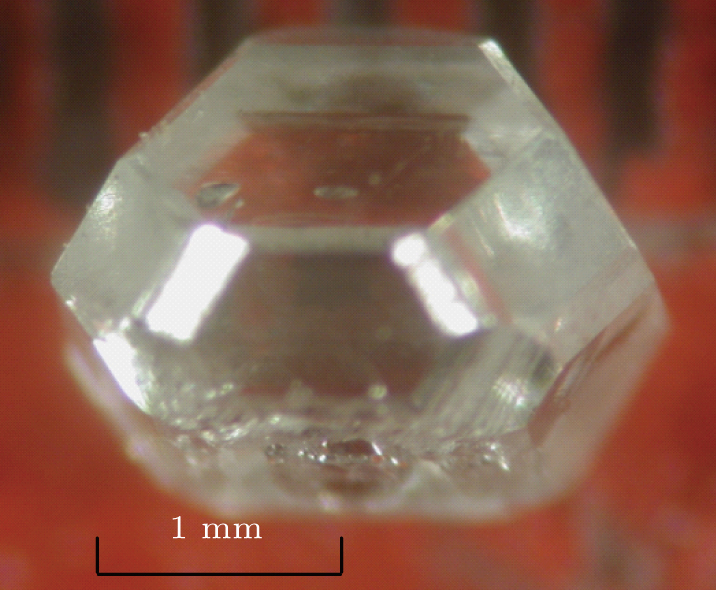
In order to synthesize high-quality colorless type-IIa large diamond without metallic inclusions or pits on its surface, we reduce the crystal growth rate and determine the content of Ti/Cu additive. The temperature gradient is reduced to about 25 °C/mm by adjusting the assembly. Finally, the growth rate drops to 1.2 mg/h. While the quantity of Ti/Cu additive is 1.8 wt%, the size of the superior diamond reaches 2.0 mm with a growth time of 12 hours. As shown in Fig.
According to the literature,[20] the Nc in type-Ib large diamond of FeNiCo as catalyst is less than that in diamond of NiMnCo as catalyst. In order to investigate whether the catalyst composition has any influence on the efficiency in eliminating nitrogen, the catalyst component is replaced by FeNiCo alloy. The Ti/Cu quantity is changed to synthesize the type-IIa large diamond. In order to facilitate comparison and analysis, the quantity of Ti/Cu ranging from 0.5 wt% to 2.0 wt% was added in FeNiCo alloy to synthesize diamonds, respectively. The growth time and temperature of diamonds are 3 hour and 1280 °C, respectively. The temperature gradient is adjusted to 33 °C/mm and the growth rate of diamonds is controlled to about 1.8 mg/h. The experimental results are shown in Table
| Table 2.
Results of diamond growth with different quantities of Ti/Cu in FeNiCo. . |
In order to determine the Nc content values in diamonds with the different quantities of nitrogen getter in FeNiCo catalyst, all synthetic diamonds in Table
It can be seen in Table
By adjusting the assembly and reducing the growth rate (1.6 mg/h), the type-IIa large diamond is synthesized in a CHPA for 12 hours (Ti/Cu for 1.6 wt%) as shown in Fig.
First, without considering the quality of crystal, it can be shown from Tables
It can be explained that the different ability to melt soluble nitrogen leads to the different efficiencies on eliminating nitrogen of Ti/Cu in both catalysts. According to the literature,[3] outer electronic shell structures of Fe, Co, Ni are 3d64s2, 3d74s2, and 3d84s2, respectively, and the numbers of these electrons in the d shell increase gradually. The lower the electron number is in the d shell, the smaller the atomic number is in the row elements of the periodic table. Therefore, nitrogen is easier to enter into, to dissolve and to stay in catalytic melt of the Fe base than that of the Ni base, and it is hard for nitrogen impurities to enter into diamond with the Fe base as catalyst. According to this theory, the ability of metal to dissolve nitrogen should be in the sequence of Fe>Co>Ni.[15] So, the Nc content in diamond with Fe-base as catalyst should be lower than in diamond with Ni-based as catalyst. This shows that the Nc content in diamond with FeNiCo as catalyst is lower than that with NiMnCo as catalyst without nitrogen getter.
Without nitrogen getter, the Nc content in diamond with NiMnCo as catalyst is 430 ppm but the Nc content is only 140 ppm in diamond with FeNiCo as catalyst according to the report.[20] This is consistent with the above discussion and analysis. More content of Ti/Cu additive is required in NiMnCo catalyst than that in FeNiCo to synthesize type-IIa diamond. Metal inclusions appear easily in diamond as the content of Ti/Cu increases.[3,10] Therefore, our study results confirm that the FeNiCo catalyst is more suitable than the NiMnCo catalyst for growing high-quality colorless type of IIa large diamond.
In order to grow high-quality type-IIa crystals with Ni-based and Fe-based catalyst, respectively, the process of large diamond should be optimized. By choosing the right amount of Ti/Cu and reducing the growth rate, type-IIa high-quality diamond crystals (Figs.
| Table 3.
Different efficiencies on eliminating nitrogen of Ti/Cu in both catalysts. . |
The FTIR absorption spectra of diamond samples in Figs.
It can be seen from Table
Therefore, our study results confirm that the FeNiCo catalyst is more suitable than the NiMnCo catalyst for growing high-quality colorless type-IIa large diamond.
According to the above results and analyses, FeNiCo alloy, which is suitable for synthesizing type-IIa diamond is selected as the catalyst. Therefore, using FeNiCo as the catalyst and Ti/Cu as the nitrogen getter, a high-quality type of IIa large diamond crystal with about 5 mm in size is synthesized after the growth time has extended to 50 hours. Then the colorless type-IIa large diamond without inclusion or pit is obtained as shown in Fig.
With adopting Ti/Cu as the nitrogen getter in NiMnCo or FeNiCo catalyst, large diamonds are grown by TGM under HPHT. Conclusions can be drawn as follows. With adopting Ti/Cu as the nitrogen getter in NiMnCo or FeNiCo catalyst, high-quality colorless type-IIa large diamonds are obtained. The efficiency on eliminating nitrogen of Ti/Cu in the FeNiCo catalyst is better than in NiMnCo. With adopting Ti/Cu as the nitrogen getter in the FeNiCo catalyst, high-quality colorless type-IIa large diamond with 5 mm in size is obtained.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] |