† Corresponding author. E-mail:
Project supported by the National Basic Research Program, China (Grant Nos. 2016YFA0202300 and 2016YFA0202302), the National Natural Science Foundation of China (Grant Nos. 61527817, 61335006, and 61378073), and the Beijing Municipal Science and Technology Committee, China (Grant No. Z151100003315006).
A fluorescent probe for the sensitive and selective determination of copper ion (Cu2+) is presented. It is based on the use of tungsten disulfide quantum dots (WS2 QDs) which is independent of the pH of solution and emits strong blue fluorescence. Copper ions could cause aggregation of the WS2 QDs and lead to fluorescence quenching of WS2 QDs. The change of fluorescence intensity is proportional to the concentration of Cu2+, and the limit of detection is 0.4 μM. The fluorescent probe is highly selective for Cu2+ over some potentially interfering ions. These results indicate that WS2 QDs, as a fluorescent sensing platform, can meet the selective requirements for biomedical and environmental application.
The detection of heavy metal ions in the areas of biological, chemical and environmental systems is very important, owing to their deleterious effects on human health.[1–3] The copper ion (Cu2+), ranking behind zinc and iron, the third most abundant essential transition metal ion in the human body, plays a critical role in various biological processes.[4–6] The U.S. Environmental Protection Agency (EPA) and the World Health Organization (WHO) have set the limits of Cu2+ in drinking water to be 1.3 mg·L−1 (∼ 20 μmol·L−1) and 2.0 mg·L−1 (∼ 30 μmol·L−1), respectively.[7–9] The intracellular Cu2+ level in cellular homeostasis is directly related to the functions of proteins and various neurodegenerative diseases such as Menkes syndrome, Alzheimer’s, Wilson’s diseases, familial amyotrophic lateral sclerosis and Parkinson’s.[10–13] Up to now, many research methods have been developed for the accurate determination of copper. The detection range is about 1.0 μM–4.0 μM.[14] In order to control the level of Cu2+ present in the environment and the ecosystem, there is a great demand for a reliable, simple and sensitive analytical method of detecting Cu2+ ions.
Since the discovery of graphene, great attention has been paid to two-dimensional (2D) materials comprised of layered transition metal dichalcogenides (TMDs) due to the high specific surface areas and outstanding electronic properties which are important for sensors, transistors, catalysis and energy storage applications.[15–30] Among the various types of 2D materials, tungsten disulfide (WS2) has drawn a notable amount of interest in its ability to form monolayers. WS2, with graphene-analogous crystal structure, is built up of W atoms sandwiched between two single layers of sulfur atoms though van der Waals forces and can be easily exfoliated along the layer plane.[31–33] Recent studies have reported that the band-gap of WS2 can be transformed from an indirect gap (1.3 eV for bulk form) into a direct gap (∼ 2 eV for monolayer) when bulk WS2 is thinned to the layer thickness.[34,35] This transition gives rise to the giant enhancement of the photoluminescence efficiency in WS2 monolayer.
Currently, some efforts have been made to synthesize photoluminescent WS2 materials and the methods can be divided into three ways including exfoliation of bulk crystals, substrate growth by chemical vapor deposition (CVD) and colloidal synthesis.[36–39] Compared with the latter two methods, the exfoliation of bulk materials not only has the advantages of time-saving, simplicity and no need for expensive instruments but also may introduce a larger perimeter–area ratio and more defects, which renders WS2 excellent photoluminescence due to quantum confinement effect and edge effect.[40] WS2 QDs would aggregate in the presence of Cu2+ because of electrostatic interactions, leading to the formation of WS2 QDs-Cu2+ complex and fluorescence quenching. Herein, the probe will provide a sensitive and selective determination of copper ions in water samples.
Transmission electron microscopy (TEM) images were achieved by a JEM-1400 operating at 120 kV. The optical absorption spectrum of WS2 QDs was obtained by a UV-3101 scanning spectrophotometer (Shimadzu, Japan) at room temperature. Fluorescence intensity was recorded on the luminescence spectrometer (Fluorolog-3). The x-ray photoelectron spectroscopy (XPS) measurement was carried out on PHI Quantera (PHI, Japan), the binding energy was calibrated with C1s=284.8 eV. Microplate reader (Thermo Scientific Multiskan MK3, Shanghai, China)
WS2 QDs were produced using a simple liquid exfoliation of bulk crystals. Briefly, 50 mg of WS2 powder (Alfa Aesar) was added to 30 mL of distilled water in a 50-mL beaker and sonicated in an ultrasonic cell disruptor (400 W) for 4 h. Then, the precipitation with 30-mL NMP (1-methyl-2-pyrrolidinone) was sonicated in an ultrasonic bath continuously for 3 h. After that, the solution was centrifuged (9000 rpm, 30 min) and filtrated to remove large WS2 sheets. Then the suspension was evaporated and re-dissolved in 50-mL water and stored at 4 °C.
The growth inhibition of the WS2-treated HepG2 cells was measured using an MTT assay. The cells were seeded into 96-well cell culture clusters (Corning, NY, USA) at a density of 6×103 cells per well. After a 12-h incubation, the cells were treated with WS2 for 6 h to 48 h. Thereafter, the cells were rinsed twice with PBS and incubated with 100 μL of a 0.5-mg/mL MTT solution at 37 °C for 4 h. After removing the supernatant, the formazan crystals formed by the MTT were dissolved with 150 μL of DMSO. The absorbance (A) at 490 nm was measured using a microplate reader. The cell viability was calculated from the following formula:
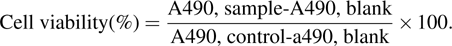 |
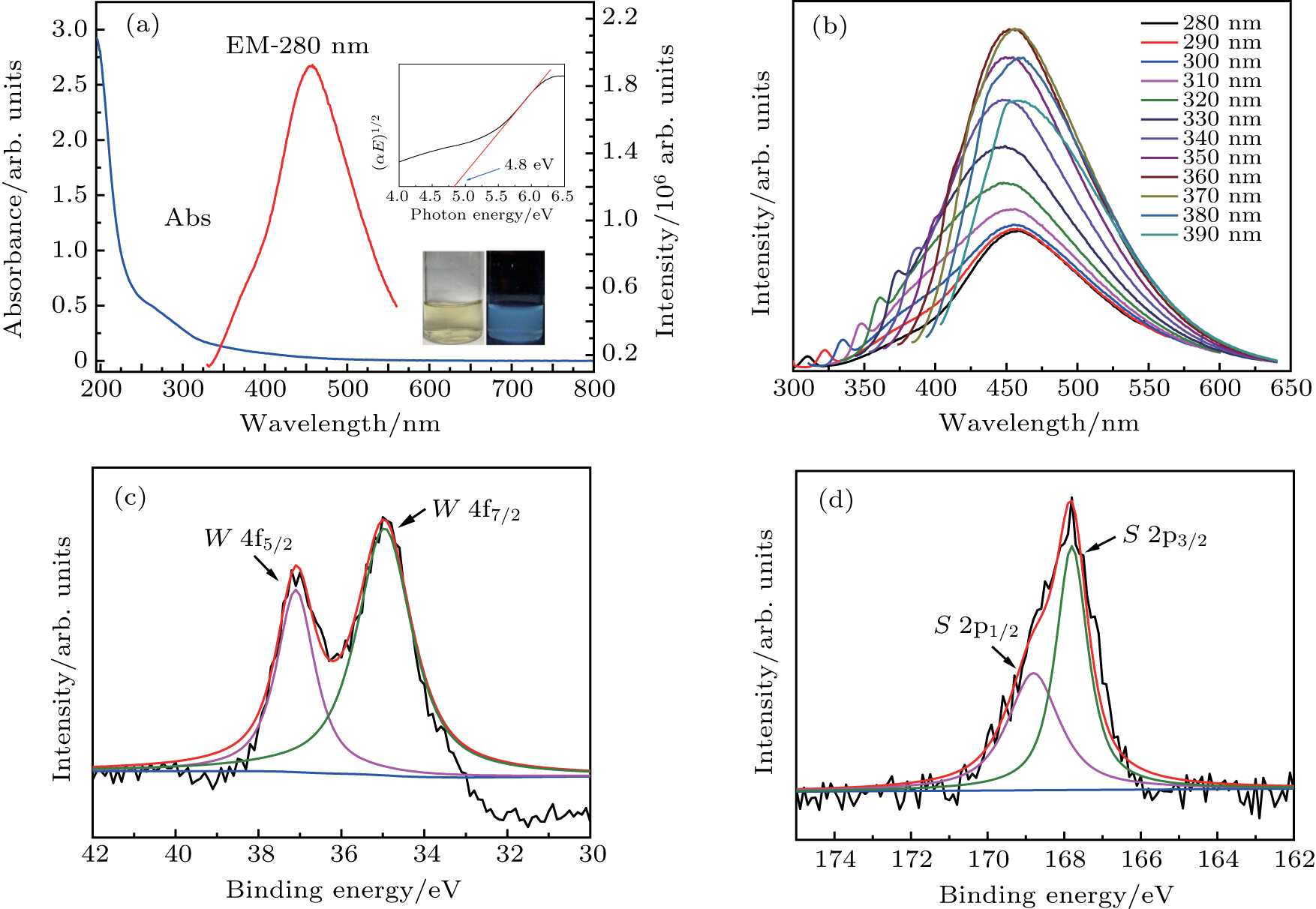
The UV–vis absorption (blue line) and fluorescence emission spectra (red line) of WS2 QDs are shown in Fig.
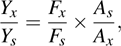 |
where Y is the quantum yield, F is the measured integrated emission intensity, and A is the optical density. The subscript “s” refers to quinine sulphate, “x” denotes WS2 QDs.
To further investigate optical properties, PL emission spectra of WS2 QDs are recorded under various excitations. As shown in Fig.
To further explore the chemical property and composition of the WS2 QDs, XPS characterization is carried out. As shown in Fig.
As shown in scheme 1 (see Fig.
 | Fig. 2. (color online) Illustrations of the preparation scheme of WS2 QDs and the detection of copper ions. |
To investigate sensitivity of the sensor, Cu2+ with concentrations ranging from 0 μM to 200 μM are analyzed by WS2 QDs under 365-nm excitation wavelength. Figure
In order to study the practical applicability, the effect of pH on the fluorescence response of WS2 QDs to Cu2+ is investigated. The experiments are carried out at a pH range from 2 to 12, with a concentration of WS2 QDs fixed at 160 μg·ml−1 and 365-nm excitation wavelength (Fig.
To assess the selectivity of Cu2+ detection, the PL response of the probe to potentially interfering substances (K+, Na+,Ca2+,Ba2+,Al3+,Fe3+,Ni2+,Ag+,Hg2+,Zn2+,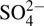
In this paper, a simple but effective fluorescent probe, WS2 QDs, is prepared for Cu2+ detection. The probe is independent of the pH of solution and hardly interfered with by other ions, upon excitation at 365 nm. The detection limit is estimated to be 0.4 μM, which is much lower than the maximum level (∼ 20 μM) set by the U.S. Environmental Protection Agency (EPA). These results indicate that WS2 QDs could meet the requirements for biomedical and environmental application.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] |