† Corresponding author. E-mail:
A stable and insoluble V2O5·nH2O/tetra-n-butyl titanate (TBO) hybrid xerogel was synthesized by the sol–gel method. This novel material proved to be an efficient absorbent with an absorption capacity of 179 mg·g−1 for Rhodamine B (RhB) in water due to its unique layered structure, which can effectively accommodate RhB molecules between its layers as demonstrated by XRD and FTIR spectroscopic analyses.
Layered materials like clays, bentonite, saponite, montmorillonite, and layered double hydroxides (LDH) exhibit excellent intercalation properties due to their ion-exchange abilities and their flexible interlayer spacing that can accommodate guest molecules between the layers without affecting other structural features.[1–6] Vanadium pentoxide (V2O5) xerogels have a layered texture similar to that of clays. Their composition can be denoted as V2O5 · nH2O, where n represents the number of water molecules that reside between adjacent vanadium oxide layers. The interlayer spacing d between the bilayer slabs is highly sensitive to the water content, and expand or contract based on the intercalation or extraction of water molecules.[7] In contrast to clay minerals, which have intrinsic metal cations for exchange, V2O5 · nH2O xerogels have only dissociable H2O molecules, and are therefore acidic in nature.[8] and show a large cation exchange capacity of ∼ 0.33 for monovalent cations.[9] V2O5 xerogels also offer a prodigious capacity for intercalating atoms as well as molecules of organic[10–13] and inorganic[9–14] substances. This property makes V2O5 · nH2O xerogels different from three dimensional crystalline V2O5, which can only accommodate metal ions. Their easy synthesis, the abundance of the source materials and high intercalation capacity are the key factors that have marked V2O5 xerogels as suitable host materials for intercalation chemistry.[15]
The intercalation of organic dyes into the lamellar structure of a V2O5 xerogel for photoactive and lasing purposes has been reported by several authors.[16–18] But few reports are found in the literature on employing V2O5 · nH2O xerogels as an absorbent material for the removal of organic dyes from waste water.[19,20] V2O5 · nH2O xerogels exfoliate in water in a manner similar to clays, and form brown coloured colloidal suspensions that limit their use in decolourizing/cleaning of aqueous media. To effectively utilize the intercalation properties of this novel material to remove dyes from waste water, there is a need to develop a hybrid V2O5 xerogel that maintains its intercalation capacity while remaining insoluble in aqueous solutions.
It has been reported that an insoluble xerogel, characterized as V2O4.8 · xH2O, can be synthesized by the reduction of fresh decavanadic acid with a reducing agent such as alcohol, acetone, hydrazine, etc.[21] Insoluble xerogels can also be prepared by the irreversible intercalation of tetrathiafulvaline (TTF) into V2O5·1.6H2O in an ethanol/water mixture that acts as a solvent.[22] It was hypothesized that the TTF stabilizes the host V2O5 xerogel, and might form reduced vanadium oxide xerogels (V2O4.8−4.9 · nH2O) that contain an abundant amount of water in a quite stable form. Furthermore, layered and stable vanadium metal oxide xerogels intercalated with tetravalent ions, with compositions V1.67M0.33O5±δ · nH2O (M = Ti or Mo), have been prepared by the sol - gel method.[23] In another study, vanadium titanium oxide xerogels were formed by the sol–gel method using V2O5 and TiH2 as starting materials. The composition and structure of the above solid solutions depend upon their contents: V2−yTiyO5−δ · nH2O (0 < y < 1.33, layered structure) and Ti1−yVyO2+y · nH2O (0 < y < 0.25, anatase structure).[24] While V2−yTiyO5−δ ·nH2O forms stable gels, the stacking order of the layers was found to be affected by an increase in the Ti content.
In this study, an insoluble hybrid V2O5 xerogel is prepared by the reduction of a V2O5 · nH2O xerogel using a solution of tetra-n-butyl titanate (TBO) mixed with ethanol. This novel material is proposed as an efficient absorbent for the toxic cationic dye rhodamine B (RhB) in aqueous solutions.
The vanadium oxide xerogel (V2O5 · nH2O) was synthesized by the sol–gel method, as described in previous literature.[25–27] In a typical synthesis, commercially purchased orthorhombic V2O5 powder (99.6%, Sigma Aldrich) was added into distilled water and mixed well to prepare a 0.05 M solution. Then, 15 mL of H2O2 (30 wt %) was added drop-wise to the solution and stirred for 1 h, leading to the formation of a reddish-brown sol. This was heated at 80 °C for 9 h to evaporate the water slowly. The final product was a reddish brown solid, which was ground into a fine powder for further use. In addition, 0.2 g of TBO (97%, Sigma Aldrich) was slowly dropped into 10 mL of anhydrous ethanol (99.7%) to form a transparent solution. Next, the V2O5 xerogel powder (0.2 g) prepared above was dispersed in the TBO/ethanol solution by ultrasonication for 30 min. The solution was kept overnight to dry under ambient conditions. A light green coloured hybrid xerogel was obtained after the drying step, which was again ground to a fine powder with the help of a spatula.
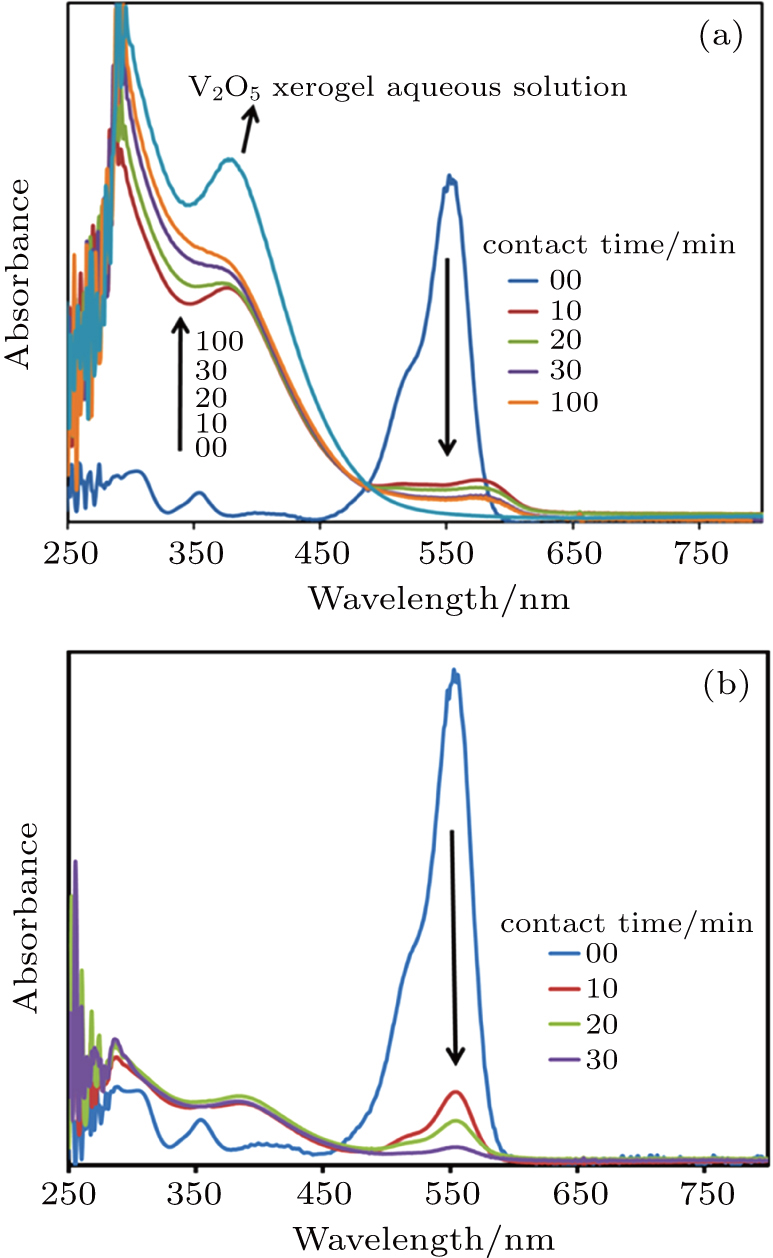
Absorption spectroscopy was performed by considering the RhB dye as the absorbate and the hybrid xerogel prepared above as the absorbent. A RhB stock solution (120 mg·L−1) was initially prepared. It was diluted further to 12 mg·L−1 by adding deionized (DI) water. The hybrid xerogel (10 mg) was added into 30 mL of this diluted RhB solution. Experiments were performed in a dark chamber to eliminate the effect of light. The mixed solution was stirred via a magnetic stirrer at 100 rpm to facilitate mass transfer. At intermittent time intervals, ∼ 4 mL of the sample solution was extracted and centrifuged at 14000 rpm for 7 min to separate the absorbent from the solution. The absorption spectrum of the supernatant solution was then analysed by using an Agilent UV–Vis spectrophotometer 8745. A calibration curve of the absorbance versus RhB concentration was established by preparing standard RhB aqueous solutions and measuring their absorption peak at 553 nm (see Fig.
Nitrogen physic-sorption measurements were conducted on a Micromeritics Tristar 3000 instrument. Surface areas and pore sizes were calculated from the isotherms by following the Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods, respectively. The zeta potential of the hybrid xerogel was measured on a Malvern Zetasizer Nano ZS.
Structural analysis of the xerogel was carried out using a D2 Phaser Bruker x-ray diffractometer (XRD). The 2θ angle range was set between 5° and 45°. XRD patterns at low angles, i.e. starting from 2°, were obtained by using a D8 Advance Bruker XRD. Both XRDs were used in locked couple 2θ/θ mode with Cu Kα radiation. A Perkin Elmer Spectrum 100 FTIR spectrometer was used in the attenuated total reflectance (ATR) mode to investigate the chemical structure of the hybrid xerogel. The FTIR spectra were obtained in the wave number range of 400–4000 cm−1, with a resolution of 4 cm−1.
Figure
 | Fig. 2. (color online) Images of RhB aqueous solutions stirred with the pure V2O5 xerogel (a), and hybrid V2O5 xerogel (b) at successive time intervals. |
When the RhB aqueous solution was mixed with the pure and hybrid xerogels, the pH values of the solutions were noted as 3.4 and 4, respectively. The point of zero charge (PZC) for the V2O5 xerogel is reported as approximately pH 2.[8] At the PZC, a substance is considered to be neutral. At pH>PZC, a substance exhibits anionic behaviour. In the present study, the zeta potential of the pure and hybrid xerogels at their respective pH values was calculated to be in the range of −24 mV to −35 mV. This indicates that the xerogel particles have negatively charged surfaces in the pH range in question. In an aqueous solution, RhB can exist in three different ionic forms, i.e. protonated RBH+, neutral zwitterionic RhB± and colourless lactone. The presence of these forms depends on the pH, concentration and temperature of the environmental solution (prototropic forms of RhB are shown in Fig.
The average pore diameter (dpor), specific surface area (SBET) and average pore volume (Vpor) of the pure xerogel were calculated by using the BET and BJH methods, and were found to be 33.8 nm, 1.25 m2·g−1 and 4.17×10−5 m3·g−1, respectively. The addition of TBO into the V2O5 xerogel induced a dramatic increase in the SBET and Vpor values to 27.57 m2·g−1 and 3.72 × 10−4 m3·g−1, respectively. Whereas the pore diameter for the hybrid xerogel was decreased to 7.25 nm, but the number of pores was increased as observed by increase in pore volume. The SBET for the hybrid xerogel was more than 22 times larger than that of the pure xerogel. The significant increase of the surface area and pore volume of the hybrid xerogel compared to the pure xerogel indicates that the former possess a more hollow structure and provides more reaction sites for the target dye molecules. The pore diameter of xerogel is sufficient for the adsorption of monomers of RhB at the tested pH of aqueous solution. Moreover, The UV–Vis spectroscopy results confirm that the hybrid xerogel has more absorption capacity for the RhB dye than the pure xerogel. While the pore diameter of the hybrid xerogel was smaller at 7.25 nm, the number of pores was larger, as indicated by the increase in the total pore volume. Furthermore, the pore diameter of the xerogel is sufficient for the adsorption of RhB monomers at the measured pH of the aqueous solution. It is speculated that the absorption of RhB by the hybrid xerogel takes place in two steps: initially the RhB is adsorbed through surface diffusion over the V2O5 hybrid xerogel, and secondly, the dye monomers intercalate between the layers of the xerogel.
To investigate the effect of different initial concentrations and absorbent dosages on the RhB absorption capacity of the hybrid V2O5 xerogel at a fixed pH = 4, a series of experiments were performed at room temperature. RhB aqueous solutions with a volume of 50 mL and initial concentrations 36, 48, 60, 72, 84, and 120 mg·L−1 were prepared. A fixed amount of the hybrid xerogel (30 mg) was added to each solution, which was then agitated in a shaker at a speed of 200 rpm for 24 h. Once the equilibrium state was reached, the amount of RhB absorbed by a unit mass of the absorbent qe (mg·g−1) was calculated by the following relation:
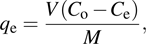 |
where V is the volume of the solution, M is the mass of absorbent, Co is the initial concentration, and Ce is the concentration of the RhB at equilibrium time t.
The uptake of RhB increases linearly from 58 mg·g−1 to 134 mg·g−1 for initial dye concentrations in the 36 mg·L−1 to 84 mg·L−1 range, but starts to deviate from the linear trend when the dye concentration is increased further to 120 mg·L−1, as shown in Fig.
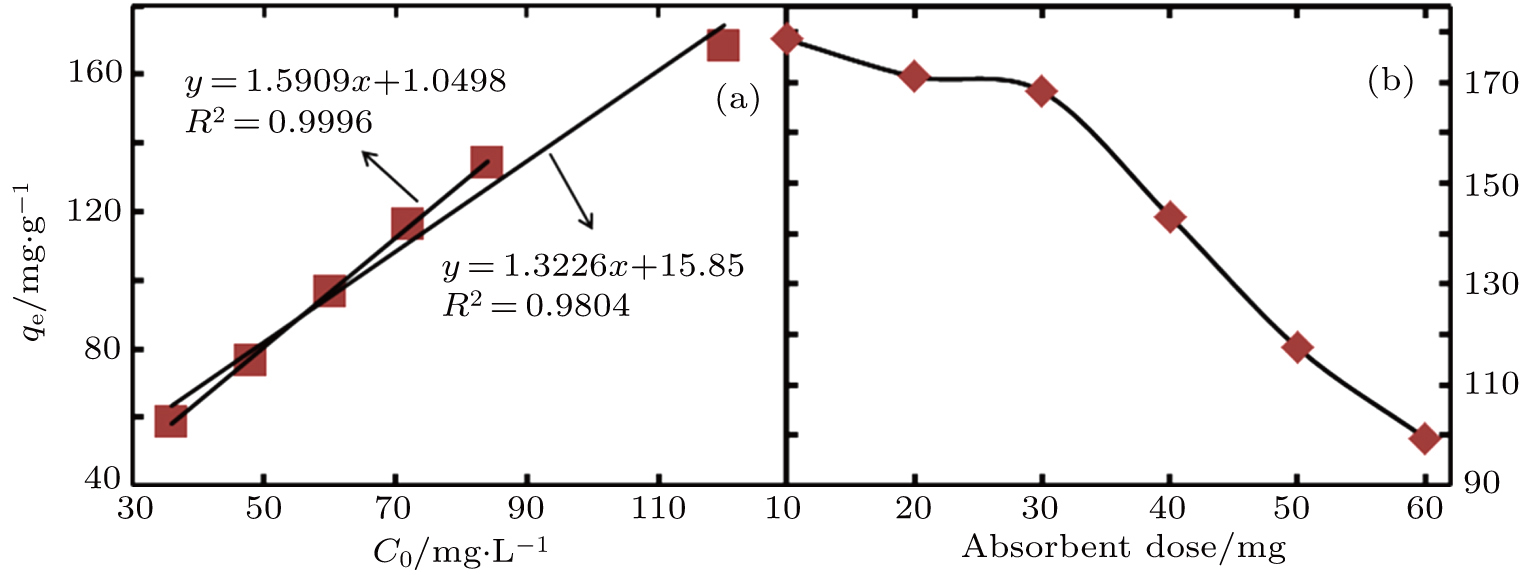 | Fig. 3. (color online) The effect of initial dye concentration (a) and absorbent dose (b) on RhB uptake by the hybrid V2O5/TBO xerogel. |
It must also be noted that the dose of absorbent in a RhB solution also affects its absorption capacity. For a fixed initial dye concentration of 120 mg·L−1, the absorbent amount was varied from 10 mg to 60 mg. In this case, the uptake of RhB was observed to be in the range from 179 mg·g−1 to 99 mg·g−1, respectively (Fig.
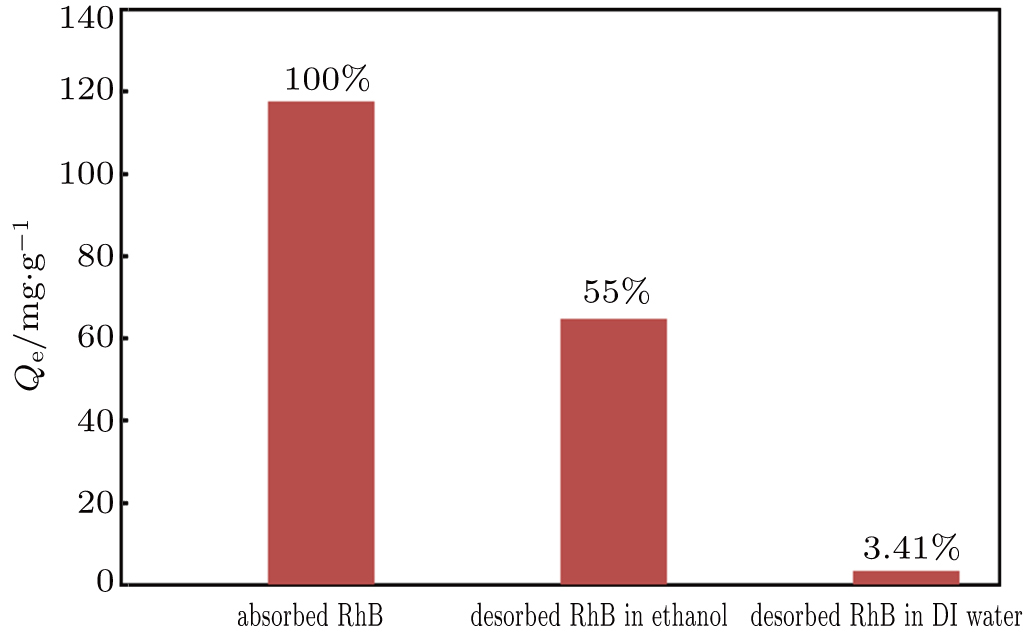
At the equilibrium time of 24 h, the hybrid V2O5 xerogel had absorbed 117 mg·g−1 of RhB from the concentrated RhB solution (120 mg·L−1). The RhB-loaded xerogel in the solution was allowed to settle for 2 h after absorption. The supernatant was carefully extracted using a set of transfer pipettes, and the precipitates were dried in an oven at 50 °C for 2 h. Two solvents, DI water and ethanol, were used in the desorption study. The solutions were gently stirred using a magnetic stirrer at 100 rpm for 24 h in a dark chamber. As shown in Fig.
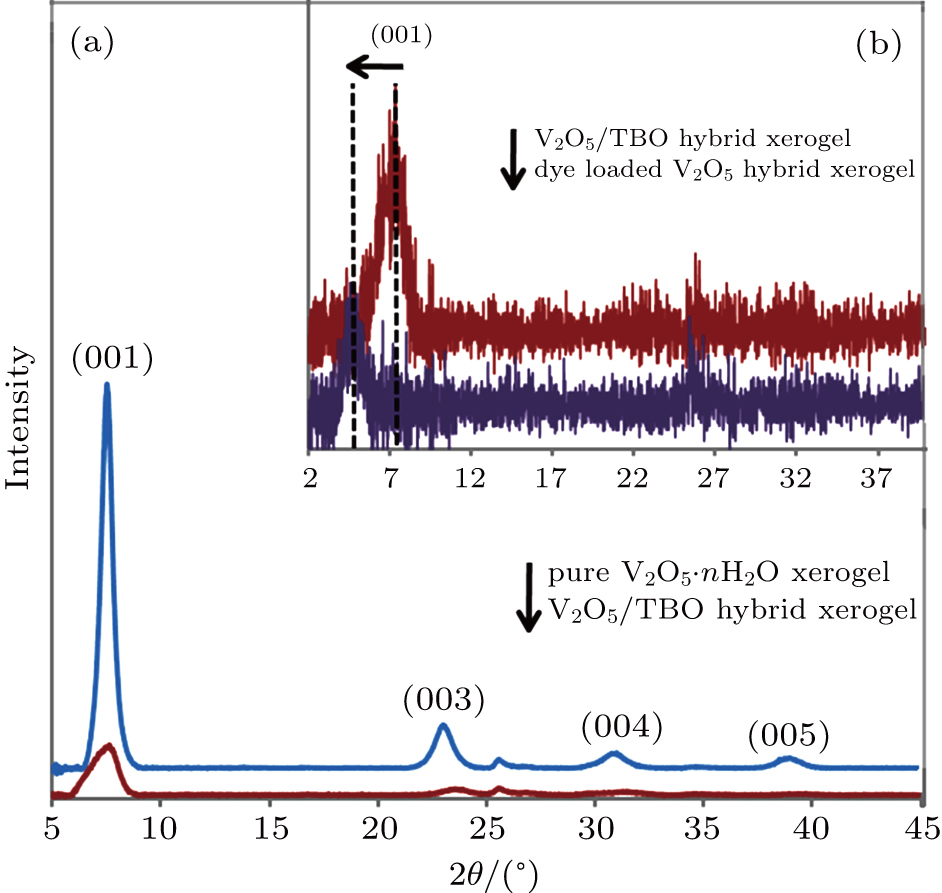
The XRD patterns reflect the well-defined lamellar structure of the pure V2O5 xerogel in the c direction, as indicated by the strong (001) peak at 2θ = 7.47° and similar reflections of (003), (004) and (005) at higher angles (Fig.
The substitution of titanium for vanadium in the V2O5/TBO hybrid xerogel destroys the stacking order of the lattice in the c direction, as shown by a decrease in the intensity of the 00l peaks.[24] Moreover, compared to the pure xerogel, the interlayer spacing d decreased from 11.76 Å to 11.58 Å in the hybrid V2O5/TBO xerogel. This indicates that during dispersion of the V2O5 xerogel in the TBO/ethanol solution, loosely bonded water molecules were ejected from the interlayer regions of the xerogel, while Ti4+ ions were intercalated between the layers to form V–O–Ti layers.[23] It is known that guest species do not always change the interlayer spacing or improve the stacking order. Instead, such changes depend on the chemical nature of the guest and host species, as well as the reactions taking place between them.[32] A redox intercalation, which reduces V5+ to V4+ or forms VO2+ ions in the xerogel, can be suggested in this case. The change in the xerogel colour from brown to green is an indication of the reduction process, as shown in Fig.
The XRD pattern in Fig.
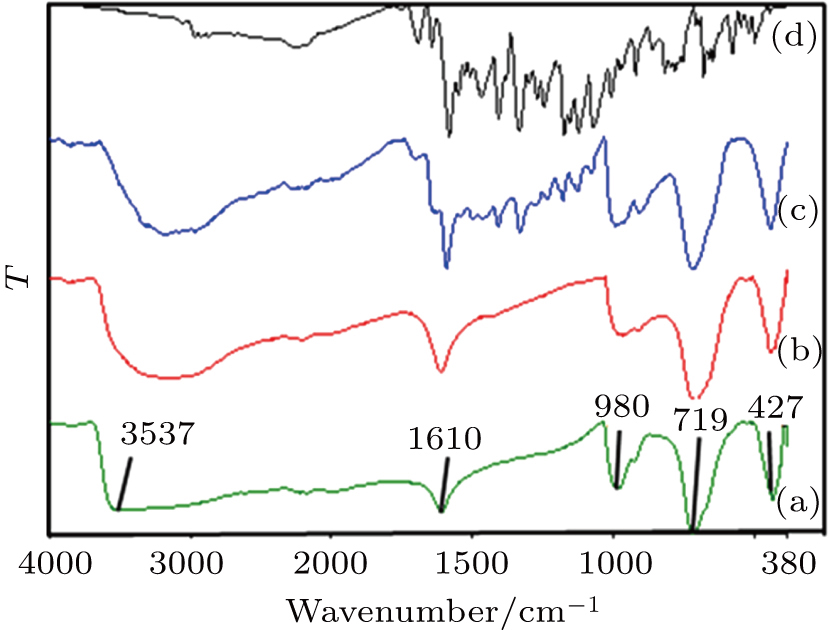
The FTIR results of the pure vanadium oxide xerogel showed a broad stretching band υs at 3600–2500 cm−1 due to O–H ions (Fig.
The insoluble V2O5/TBO hybrid xerogel developed in this study showed a remarkable absorption capacity for RhB, reaching 179 mg·g−1. The sorption mechanism, predominantly driven by electrostatic forces between the positively charged RhB molecules and negatively charged hybrid xerogel surfaces, was proposed to explain this phenomenon. FTIR analysis proved the reduction of the V2O5 ·nH2O xerogel after being treated with TBO, as well as the intercalation of the RhB molecules within the lamellar structure of the hybrid xerogel. The increase in d-spacing in the hybrid xerogel from 11.58 Å to 19.24 Å after absorption of RhB (evaluated by XRD) further confirmed this phenomenon.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] |