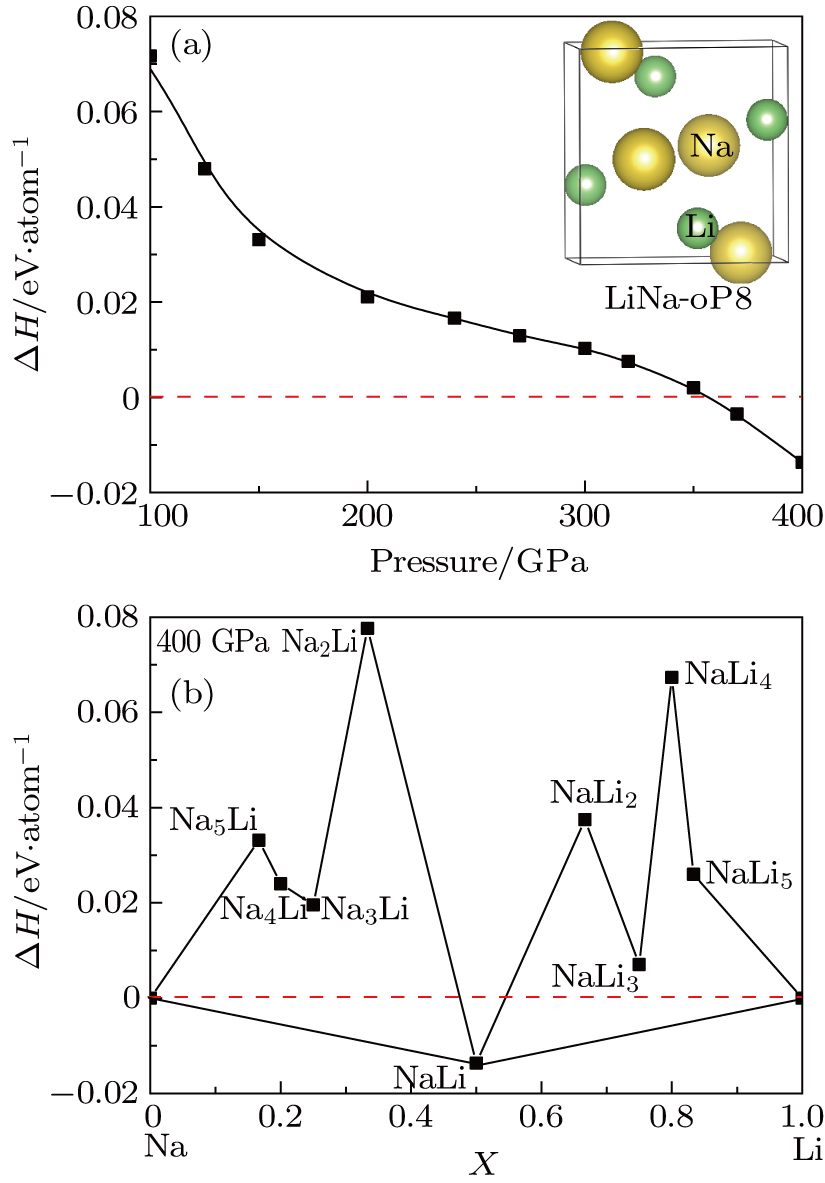
Predicted novel insulating electride compound between alkali metals lithium and sodium under high pressure
(color online) (a) Pressure dependent formation enthalpy of Li–Na calculated by PBE functional. Inset: the crystal structure of LiNa-oP8. (b) Enthalpies of formation of LimNan (m = 1, n = 2–5 and n = 1, m = 2–5) with respect to decomposition into elemental Li and Na at 400 GPa. The abscissa x is the fraction of Na in the structures. For elemental Li, the cmca-24 (80 GPa–185 GPa), cmca-56 (185 GPa–269 GPa) and p42mbc (> 269 GPa) structures[