† Corresponding author. E-mail:
The stability, electronic structures, and mechanical properties of the Fe–Mn–Al system were determined by first-principles calculations. The formation enthalpy and cohesive energy of these Fe–Mn–Al alloys are negative and show that the alloys are thermodynamically stable. Fe3Al, with the lowest formation enthalpy, is the most stable compound in the Fe–Mn–Al system. The partial density of states, total density of states, and electron density distribution maps of the Fe– Mn–Al alloys were analyzed. The bonding characteristics of these Fe–Mn–Al alloys are mainly combinations of covalent bonding and metallic bonds. The stress-strain method and Voigt–Reuss–Hill approximation were used to calculate the elastic constants and moduli, respectively. Fe2.5Mn0.5Al has the highest bulk modulus, 234.5 GPa. Fe1.5Mn1.5Al has the highest shear modulus and Young’s modulus, with values of 98.8 GPa and 259.2 GPa, respectively. These Fe–Mn–Al alloys display disparate anisotropies due to the calculated different shape of the three-dimensional curved surface of the Young’s modulus and anisotropic index. Moreover, the anisotropic sound velocities and Debye temperatures of these Fe–Mn–Al alloys were explored.
Iron, manganese, and aluminum are the main components of low density, high strength steel. The Fe–Mn–Al alloys have recently attracted much attention because these alloys have excellent corrosion resistance, outstanding mechanical properties, and low density.[1,2] In addition, the Fe–Mn–Al system is known as an effective substitute for stainless steel at high temperature oxidation resistance.[3] The Fe–Mn–Al alloys also provide excellent mechanical properties and can serve as heat-resistant steel, cryogenic steel, magnetic material, and nonmagnetic stainless steel.[4] Earlier research shows the Fm3m type DO3 [(Fe,Mn)3Al] phases distributing in ferrite in the Fe–Mn–Al–C steel; the ordered phases have a reinforcement effect in the Fe–Mn–Al–C steel.[5] Therefore, we here investigate the stability, electronic structures, and mechanical properties of the Fe–Mn–Al system. Some Fe–Mn–Al alloys have been investigated by experiments and theory in recent years. Yang et al.[6] studied the tensile deformation of low density Fe–Mn–Al–C steel. Zhang et al.[7] investigated the elastic properties of ferromagnetic body-centered cubic Fe–Mn–Al alloys. Lindahl et al.[8] studied the ordering in ternary BBC alloys applied to the Al–Fe–Mn system. Umino et al.[9] studied the phase equilibria in the Fe–Mn–Al-system by the experimental determination and thermodynamics. Yan et al.[10] investigated the anomalous behavior of electrical resistivity in Fe–Mn–Al alloys. Rico et al.[11] studied the magnetic and structural properties of mechanically alloyed Fe0.70−xAl0.30 (x = 0.40 and 0.45) alloys. The above studies focus mostly on phase equilibria, electrical resistivity, and magnetic properties. However, the electronic structures and mechanical properties of the Fe–Mn–Al system still have not been studied systemically. In our research, we employ first-principles calculations based on density functional theory (DFT), investigating the stability, electronic structures, and Debye temperatures of Fe– Mn–Al alloys. Our aim is to obtain complete data of the mechanical properties and electronic structures of the Fe–Mn–Al system. This work will contribute greatly to designing promising high-temperature structural materials, functional materials, composite materials, and high strength materials. The calculated results will help us design different performance materials, and provide theoretical data to design new steel material for us, and so on. The properties of Mn dissolved into Fe3Al ordered phase have no related data in experiments. This work will have a certain reference value.
The crystal structures of the FexMn3−xAl system are shown in Fig.
In the CASTEP code, the elastic constants were calculated by the efficient stress–strain method based on Hooke’s law. Several different strain patterns were used to calculate the crystal structure, and the Cauchy stress tensor for each strain mode was evaluated. For a cubic crystal, the related elastic constants can be expressed as[17]
 | (1) |
The thermodynamic stability of the Fe3−xMnxAl system was evaluated by calculating the formation enthalpy and cohesive energy. In this work, in order to investigate the thermodynamic stability of the FexMn3−xAl system, the two energy formulas can be defined as
 | (2) |
 | (3) |
In this process, we can obtain the lattice parameters after optimizing the crystal structure. The lattice parameters, formation enthalpy, and cohesive energy of Fe, Mn, Al, and the FexMn3−xAl system are listed in Table
| Table 1.
The calculated lattice parameters (Å), cohesive energy Ecoh (eV/atom), and formation enthalpy ΔHr (eV/atom) of the Fe–Mn–Al system. . |
In Table
In this section, in order to analyze the chemical bonding characteristics and electronic structures of the Fe3−xMnxAl system, we report our calculations of the total density of states (TDOS), partial density of states (PDOS), and electron density distribution maps. The calculated electronic structures are shown in Figs.
 | Fig. 3. (color online) Calculated total density of states and partial density of states for Fe and Fe3−xMnxAl system. Dashed lines represent the Fermi level. |
The calculated partial density of states and total density of states of the Fe3−xMnxAl system are shown in Fig.
The calculated total electron density distribution maps and electron density difference distribution maps of the Fe3−xMnxAl system are shown in Fig.
According to the above discussion, these Fe–Mn–Al alloys have magnetic characteristics. The bonding characteristics of these Fe–Mn–Al alloys are combinations of covalent bonds and metallic bonds, also exhibiting an anti-bond effect. The interaction between Fe and Al is mainly covalent bonds. Based on the Fe (1.83), Mn (1.55), and Al (1.61) electronegativity, the electronegativity difference is small, so their covalent interactions are weak. We can conclude that these alloys have weaker covalent bonding effects. These bonding features may lead to relatively high melting points and hardness.
The Fe–Mn–Al alloys are potentially high-strength materials, so investigation of mechanical performance is very important. We calculated the elastic constants of the Fe3−xMnxAl system. The results are listed in Table
| Table 2.
Calculated elastic constants (Cij), bulk modulus, shear modulus, Young’s modulus, hardness, B/G, and Poisson’s ratio (σ) of Fe–Mn–Al alloys; the units are GPa. . |
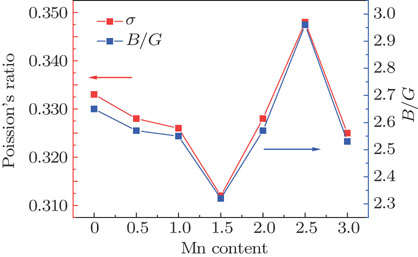
From Table
Here, the subscripts R and V indicate the Reuss and Voigt averages. BVRH, BV, andBR are the bulk moduli calculated by Voigt–Reuss–Hill, Voigt, and Reuss approximation methods, respectively. GVRH, GV, and GR are the shear moduli calculated within Voigt–Reuss–Hill, Voigt, and Reuss approximation methods, respectively.
The results of calculations and experiments are listed in Table
 | (11) |
| Table 3.
Calculated universal anisotropic index (AU), anisotropy (AB and AG), and shear anisotropic factors (A1, A2, A3) of Fe–Mn–Al alloys. . |
In order to further depict the mechanical properties of the Fe3−xMnxAl system, some elastic moduli with change to Mn atom content are curve listed in Figs.
 | Fig. 6. (color online) Variations of the bulk modulus, shear modulus, and C44 of Fe3−xMnxAl system. Note that the shear modulus is magnified by 2 times. |
All of the single crystals are actually anisotropic, therefore, a suitable parameter describing the anisotropic degree is necessary. Meanwhile, the appearance of micro cracks in materials is always connected with the anisotropy. The micro cracks often form in materials and harm the performance of the materials. Fe–Mn–Al alloys are potential high-strength materials, so the anisotropic mechanical properties of these alloys are investigated carefully. We calculated several anisotropic indexes, including the anisotropy index (AB and AG), the shear anisotropic factors (A1, A2, and A3), and universal anisotropic index (AU). The equations are as follows:[39]
 | (12) |
 | (13) |
 | (14) |
 | (15) |
In order to better depict the features of elastic anisotropy, we plotted a curved surface of three dimensions (3D). In this work, the Young’s modulus is plotted at different directions using spherical coordinates. And the directional dependence of Young’s modulus can be given by the following equations for cubic crystal:[40]
Here, l1, l2, and l3 are the directional cosines, and Sij are the elastic compliance constants. In the spherical coordinates, l1 = sin[θ]cos[ψ], l2 = sin[θ]sin[ψ], and l3 = cos[θ]. The angle between θ and ψ is defined on the horn in the spherical coordinates. The 3D curved surface of the Young’s modulus of the Fe3−xMnxAl system is shown in Fig.
The sound velocities of transverse and longitudinal modes of the Fe3−xMnxAl system are calculated using the single crystal elastic constants.[41] The sound velocities are determined by the propagation direction and symmetry of the crystal. The pure longitudinal and transverse modes are found in [100], [110], and [001] directions, and the sound propagating modes in other directions are the quasi-longitudinal or quasi-transverse waves. In the principle directions, the acoustic velocities can be calculated using the following relations:[42–44]
Here, vl is the longitudinal sound velocity; vt1 is the first transverse modes; vt2 is the second transverse modes; and ρ is the theoretical density. The calculated anisotropic sound velocities of the Fe3−xMnxAl alloys are listed in Table
| Table 4.
Anisotropic sound velocities of Fe–Mn–Al alloys; the unit of velocity is m·s−1. . |
The Debye temperature is a fundamental parameter and is related to some physical properties of materials, such as specific heat, elastic constants, and melting point.[45] It is obvious that the Debye temperature and sound velocity can be used to estimate the chemical thermal properties and chemical bond behavior of compounds. The average sound velocity (vm) and Debye temperature (ΘD) can be calculated using the following equations:[46]
 | (18) |
 | (19) |
 | (20) |
 | (21) |
We calculated the sound velocities and Debye temperature of the Fe3−xMnxAl system, and the values are listed in Table
| Table 5.
Theoretical density (ρ, kg·m−3), longitudinal sound velocity (vl, m·s−1), transverse sound velocity (vs, m·s−1), average sound velocity (vm, m·s−1), and Debye temperature (ΘD, K). . |
The stability, electronic structures, mechanical properties, and Debye temperature of these Fe–Mn–Al alloys were studied by first-principles calculations. The cohesive energy and formation enthalpy illustrate that the alloys are thermodynamically stable. We calculated the electron density distribution maps and density of states. We deduced that the bonding characteristics of the Fe–Mn–Al alloys are dominated by covalent bonds and metallic bonds. The elastic constants of these Fe3−xMnxAl alloys reflect the mechanical stability conditions, which indicate that the Fe–Mn–Al alloys are thermodynamically stable. We calculated the elastic constants (Cij), shear modulus (G), Poisson’s ratio (σ), bulk modulus (B), and Young’s modulus (E) by Voigt–Reuss–Hill (VRH) approximation. Fe1.5Mn1.5Al has the largest Young’s modulus and shear modulus. We can speculate that Fe1.5Mn1.5Al is the hardest Fe3−xMnxAl species. We calculated several anisotropy indexes to estimate the mechanical anisotropy, which together, indicate that Fe3Al has the strongest anisotropy. Young’s moduli of these Fe–Mn–Al alloys are plotted to show the anisotropy by a 3D surface contour. These alloys have strong anisotropy characteristics in Young’s modulus. The anisotropic sound velocities and Debye temperature for these alloys were also calculated. Fe1.5Mn1.5Al has the largest Debye temperature, 551.1 K, which shows that Fe1.5Mn1.5Al has stronger covalent bond features than the other alloys.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] | |
| [41] | |
| [42] | |
| [43] | |
| [44] | |
| [45] | |
| [46] |