† Corresponding author. E-mail:
In this work, the effect of electrical discharge on nitrate concentration is considered in aqueous solution. The atmospheric pressure plasma was produced by a high-voltage power supply at 27 kHz using pin-to-pin configuration. Air, argon, and argon/methane mixture were used to study the working gas effect. UV-VIS spectroscopy and ion chromatography were used to analyze the effect of the electrical discharge on nitrate concentration in deionized water. Optical emission spectroscopy (OES) was applied to diagnose active species inside and on the surface of the deionized water solution. The results of the present work showed that the atmospheric pressure electric discharge with air increases nitrate concentration while it remains constant using argon and argon/methane electrical discharges. It was also revealed that in the presence of air, the electrical discharge reduces pH, acidifying the solution and increasing solution conductivity due to production of extra nitrate ions. On the other hand, argon electrical discharge increases pH and conductivity due to production of OH- ion in water.
Atmospheric-pressure plasma processing has drawn great interest for applications such as surface cleaning, deposition, etching, sterilization, and pollution decontamination due to its low cost, high processing speed, simple system, and without any vacuum equipment.[1-9] Recently, much attention has been focused on electrical discharge process in liquid phase and many experimental investigations have been carried out to recognize production mechanism and chemical reactions between non-thermal plasma and liquid phases.[10–16] In the last few years, the use of non-thermal atmospheric pressure plasma in water has been investigated because of its importance in electrical transmission processes and its practical applications in biology, chemistry, and electrochemistry. Atmospheric pressure plasma in air contains active species such as positive and negative ions, free electrons, free radicals, ozone, and ultraviolet radiation that can be dissolved in liquid to react and form other different species. During discharge in water, hydroxyl (OH) and hydroperoxyl (HO2) radicals form in the plasma phase, dissolve in water to react and form H2O2. Generally, strong electric fields applied to water (electrohydraulic discharge) initiate both chemical and physical processes.[11,12,17–20]
In this work, we report the effects of atmospheric pressure, non-thermal plasma produced with argon, argon/methane, and air as working gases, on an aqueous solution of nitrate. The components and characteristics of solution, before and after plasma treatment, were analyzed with, UV-VIS spectroscopy (UV-2100), pH meter (Metrohm 780), conductivity meter (Jenway), and ion chromatography (Metrohm Model 850). Meanwhile optical emission spectroscopy IR-UV (SOLAR LAZER SYSTEM, S100) with scanning range from 190 nm to 1100 nm and the resolution 0.1 nm, was used to find reactive plasma species applied to water. The diffraction grating and slit width are 300 lines/mm and 20, respectively.
This paper is organized as follows: In Section
Figure
The argon (99.999%), methane (99.99%), and air were used as working gases for generating non-thermal plasma passed through the space between two powered electrodes. The atmospheric pressure plasma was generated between the two electrodes both on the water surface and inside of it close to the second powered electrode. For argon and argon/methane electrical discharges, the chamber was initially filled with the gases at atmospheric pressure and then the plasma ignited. The flow rates of argon, methane, and air were controlled with digital mass flow meter. The flow rates of argon and air gases were 600 sccm. In the case of argon/methane mixture, the flow rates of argon and methane were 300 sccm.
The effect of electrical discharge on aqueous solution of potassium nitrate was investigated in two wavelength ranges, visible and ultraviolet, with UV-VIS spectroscopy. Atmospheric pressure plasma was applied to 25 ml of KNO3 solution with different concentrations, for 13-minutes treatment. In order to find the treatment time effect, we treated the solution with constant concentration of KNO3 (80 ppm) in different times, too.
In order to investigate the plasma effect on nitrate concentration by UV-VIS spectroscopy, one pack of nitrate reagent[21] was added to 25-milliliter solution of potassium nitrate before and after applying plasma. Nitrate reagent contains Cd+2 ion and sulfonic acid. The reaction between nitrate and Cd+2 ions produces nitrites. The nitrites then react with sulfalinic acid forming a red color solution whose absorption of about 543 nm indicates the original concentration of nitrate in the solution.
The results obtained in this research are divided into three parts, air, argon, and argon/methane plasmas. Finally the results will be compared. A photograph of air, argon and argon/methane plasmas in which they were ignited on the aqueous nitrate solution is shown in Fig.
 | Fig. 2. (color online) Plasma treatment of aqueous solution of nitrate by (a) air, (b) argon, (c) argon/methane plasmas. The upper electrode and water surface are seen in each discharge. |
In this case, we prepared solutions of nitrate with concentration of 40, 80, and 100 ppm. These solutions were treated for 13 minutes, continuously. Figure
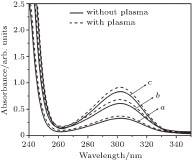 | Fig. 3. Comparison between UV-VIS spectra of nitrate solutions, curve a: 40, curve b: 80, and curve c: 100 ppm before and after applying air plasma. |
Figure
The nitrate reagent was purchased from Hach company. The reaction equations of nitrate reagent with KNO3 salt based on its specification.
Figure
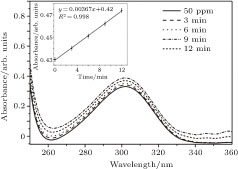 | Fig. 5. UV-visible spectra of the nitrate solution (initial concentration 50 mg/L) before and after air plasma treatment. |
Effect of duration of applying air plasma with the initial concentration of 80 ppm has been shown in Fig.
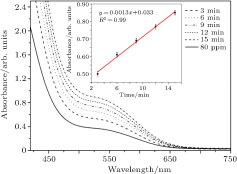 | Fig. 6. (color online) Comparison between absorption intensity, before and after applying plasma to aqueous solution of nitrate at visible range with initial concentration of 80 ppm. |
The following reactions seem to be responsible for increasing nitrate concentration in air plasma treatment.[12] First, we write the main chemical reactions in the discharge channels that happened over the liquid surface in air:[12,22–30]
NO2 molecules can be solved into the nitrate solution and initiate several chemical reactions:
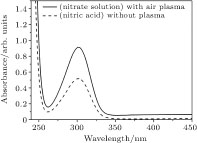
In order to confirm the bi-products, we prepared nitric acid solution using deionized water. Then, we took the absorption curve of the sample (nitric acid solution) by UV-VIS spectroscopy about 300 nm, a well-known absorption band of the nitric acid solution. It was revealed that the absorption curve of the nitric acid solution coincides with that of the treated nitrate solution samples by air plasma. This was depicted in Fig.
In Subsection
 | Fig. 8. Comparison between absorption intensity, before and after applying argon plasma to aqueous solution of nitrate at visible range at different concentrations. |
The ion chromatography was used for measuring the nitrate concentration before and after plasma processing. The initial concentration of the nitrate solution was 31.66 ppm. Argon and air plasmas were applied 12 min and 3 min, respectively. The results of the ion chromatography are shown in Fig.
| Table 1.
Comparison between concentration of nitrate solution, after applying argon and air plasmas. . |
The reactions in aqueous solution of nitrate in the presence of argon plasma can be written as[10,15,22,23,26,30,31]
Figure
 | (39) |
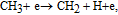 | (40) |
 | (41) |
 | (42) |
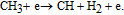 | (43) |
 | Fig. 10. Ion chromatography of nitrate solution before and after treatment by Ar and Ar/CH4 plasmas. |
| Table 2.
Comparison between concentration of nitrate solution, after applying argon and argon/methane plasmas. . |
The pH of the samples was measured before and after plasma treatment which is indicated in Fig.
 | Fig. 11. Comparison between pH of solution, after applying argon/methane, argon, and air plasmas to aqueous solution at different times. |
 | Fig. 12. (color online) Comparison between conductivity of solution, after applying air, argon, and argon/methane plasmas to aqueous solution at different times. |
As shown in Fig.
The results obtained by UV-VIS spectroscopy for air and argon plasmas processing on nitrate solution have been compared in Fig.
 | Fig. 13. Comparison between absorbance of nitrate solution, after applying argon and air plasmas at different concentrations. |
Typical V−I characteristics of our setup in the absence and presence of discharge are shown in Figs.
 | Fig. 14. (color online) Time dependence of discharge voltage and current (a) in the absence of plasma (b) in the presence of plasma. |
Figure 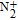
 | Fig. 15. Optical emission spectra of air plasma formed in pin-to-pin configuration, between upper electrode and the solution surface. |
Figure
 | Fig. 16. Optical emission spectra of argon plasma formed in pin-to-pin configuration, between upper electrode and the surface of solution |
Optical emission intensity of argon and argon/methane plasmas in the nitrate solution were indicated in Figs.
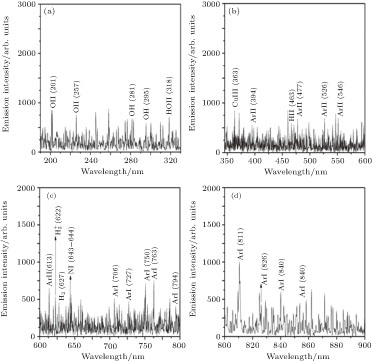 | Fig. 17. Optical emission spectra of argon plasma formed in pin-to-pin configuration in the nitrate solution. |
In this paper, the effect of non-thermal plasma on nitrate solution was investigated. The atmospheric pressure plasma was produced in a pin-to-pin configuration of the electrodes, where one of the electrodes was immersed in the nitrate solution and the other was set over the water surface. The applied voltage was 0 kV−15 kV at 27 kHz. Ion chromatography measurements revealed that the air discharge on nitrate solution increases nitrate concentration by 74%, while Ar and Ar/CH4 discharges, change nitrate concentration by less than 5%. The former was attributed to the extra nitrate production via chemical reactions initiated in the discharge channels over the nitrate solution. On the other hand, spectroscopic measurements showed that air discharge on the nitrate solution yield nitric acid in the solution. This effect could be a great challenge in water purification processes by such plasmas. Solution pH decreased from 5.3 to 2.2 after 15-min running air plasma while it was increased from 5.3 to 6.5 by Ar and Ar/CH4 discharges. The former can be attributed to HNO2, HNO3, and H+ products and the latter to the OH- ions. The conductivity of the solution was increased for all discharges that we used. In the case of air discharge, the change in conductivity was three orders of magnitudes while it increased to twice its initial value for the Ar and Ar/CH4 discharges. The results of this study can be useful in water purification by plasmas, and nitrate remediation in drinking water by such discharges.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] |