† Corresponding author. E-mail:
We experimentally fabricate a non-spherical Ag and Co surface-enhanced Raman scattering (SERS) substrate, which not only retains the metallic plasmon resonant effect, but also possesses the magnetic field controllable characteristics. Raman detections are carried out with the test crystal violet (CV) and rhodamine 6G (R6G) molecules with the initiation of different magnitudes of external magnetic field. Experimental results indicate that our prepared substrate shows a higher SERS activity and magnetic controllability, where non-spherical Ag nanoparticles are driven to aggregate effectively by the magnetized Co and plenty of hot-spots are built around the metallic Ag nanoparticles, thereby leading to the enhancement of local electromagnetic field. Moreover, when the external magnetic field is increased, our prepared substrate demonstrates excellent SERS enhancement. With the 2500 Gs and 3500 Gs (1 Gs = 10−4 T) magnetic fields, SERS signal can also be obtained with the detection limit lowering down to 10−9 M. These results indicate that our proposed magnetic field controlled substrate enables us to freely achieve the enhanced and controllable SERS effect, which can be widely used in the optical sensing, single molecule detection and bio-medical applications.
Surface-enhanced Raman scattering (SERS) is a powerful detection technique, which has the ability to fingerprint the molecule vibrational mode. It is widely used in the ultra-sensitive chemical or biomedical analysis. [1, 2] Based on the SERS phenomenon, a high-vacuum tip-enhanced Raman spectroscopy system with high spatial and spectral resolution, which allows in situ sample preparation and measurement, has been built which is beneficial for basic surface studies. Such a system shows simultaneously activated infrared and Raman vibrational modes, Fermi resonance, and some other non-linear effects.[3] The SERS happens with the aid of the metallic nanoparticles, arrays or their aggregations, where increased Raman signal arising from the enhanced surface plasmon resonance is obtained. [4–6] Two kinds of enhancement mechanisms are involved for the SERS.[7] One is the chemical enhancement caused by the charge transfer. The other is the local electromagnetic enhancement that originates from the excitation of the collective oscillations of free electrons in noble or transition metals. The extent of enhancement strongly depends on the surface morphology of SERS substrate like the size, shape and aggregation form of nanoparticles.[8–10] Much work has been carried out on the non-spherical nanoparticles, like the hierarchical nanoparticles with nanogaps and nanotips built on the nanoparticle surface [11–13], rough surface substrate of nanoarchitectures with hedgehog-shaped, cauliflower-shaped, or bunch-of-grapes-shaped surface features [14], various branched metallic architectures [15–17], large-scale monocrystalline metallic nanooctahedra [18], multifarious metallic nanoparticles architectures, etc. [19] Their SERS activities are considerably higher than those of normal spherical mono-metallic nanoparticles. [20]
One critical requirement for the surface-enhanced Raman scattering application is to design a stable and reproducible surface-enhanced Raman scattering substrate. To meet the above challenge, much effort has been made to fabricate the surface-enhanced Raman scattering substrates with the help of external field like the electric field [21], temperature, or magnetic field.[22, 23] Among them, magnetic materials with noble metals (Au or Ag) are commonly used to form composite structures. It can not only reduce the cost of noble metals but also make it easy to be separated and concentrated.[24] Several types of magneto–plasmonic hybrid nanoparticles have been reported including Co/Au core/shell nanoparticles with one-step seeding-growth,[25] and Co@Cu nanoparticles via pulsed laser dewetting method,[26] Ag/FeCo/Ag core/shell/shell nanoparticles by the hot injection method in combination with the polyol method and Au–Fe3O4 heterostructured nanoparticles.[27] Our group has synthesized the Ag–Fe3O4 nanocomposites by the redox reaction between Ag2O and Fe(OH)2 in the absence of additional reductant at a temperature of 90 °C and under atmospheric condition.[28]
Here in this study we present the synthesis and applications of novel magneto-optical surface-enhanced Raman scattering substrate comprised of non-spherical silver and cobalt nanoparticles. These materials present unique optical properties and magnetic field controllability. The surface-enhanced Raman scattering enhancement behavior is checked with different applied external magnetic fields and molecule detection concentrations. As expected, our prepared substrate provides excellent surface-enhanced Raman scattering signal, which is caused by the closed packed metallic nanoparticles induced by the magnetic field. The detailed influence of magnetic field on our prepared surface-enhanced Raman scattering substrate is clearly described in the following sections.
Cobalt nanoparticles are prepared by a very facile reduction method at ambient temperature.[29] The 0.2 g of 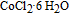
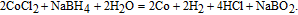 |
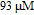
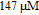
The morphology of nanoparticles is characterized with JSM-6700F field emission scanning electron microscopy (FE-SEM). JEM-100CX transmission electron micrograph (TEM) is used to characterize its morphology. Absorption spectra are taken by a UV 3150 UV-VIS-NIR spectrophotometer. Raman spectra are collected at room temperature by Renishaw-2000 Raman spectrometer (Britain) with 532-nm laser excitation. The laser is focused onto the samples from the top by an objective lens (50x, NA, 0.55). To prepare the SERS substrate, the magnetic field is initiated during the sample preparation. We firstly deposit cobalt colloid on the pre-cleaned glass slide, where different sizes of the magnetites are placed underneath the glass slid. Then mixed solution of silver colloid and test molecule is deposited. Finally, let the substrate dry under ambient conditions. All samples are used after preparation for SERS measurements. Ten Raman spectra are measured at random positions on each substrate and then averaged.
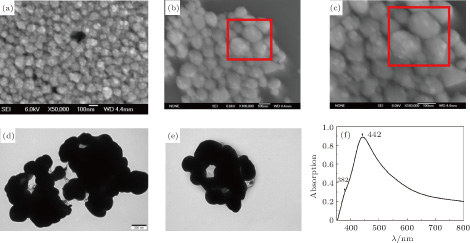
SEM images of our prepared non-spherical Ag nanoparticles with rough surface are shown in Figs.
The morphology and size of the as-synthesized Co nanoparticles are characterized by TEM as indicated in Figs.
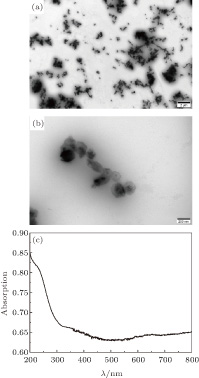 | Fig. 2. (color online) (a) and (b) TEM images of the prepared Co nanoparticles with different magnifications; (c) absorption spectrum of the non-spherical Ag colloid. |
With 10−6-M CV serving as the test molecule, SERS activity of the non-spherical Ag and Co SERS substrate is investigated in Fig.
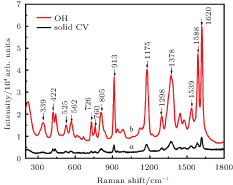 | Fig. 3. (color online) (curve a) Raman spectra of solid powder CV, and (curve b) 10−6-M CV absorbed on SERS substrates without external magnetic field. |
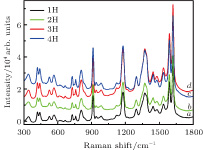
To fully explore the magnetic field controllable SERS activity, we investigate the substrate with four different magnitudes of external magnetic field. Here 1H, 2H, 3H, and 4H represent the external magnetic fields of 1500 Gs, 2000 Gs, 2500 Gs, and 3500 Gs, respectively. Ferromagnetic Co nanoparticles have uniform vector magnetization due to the superparamagnetic properties. The direction of its magnetization is determined by the applied magnetic field. When the difference between the maximum and minimum values of free energy per unit volume is very large in comparison with the thermal energy, we may ignore thermal agitation and calculate the static magnetization at each magnetic field. Any aggregation in the nanoparticle solution before the initiation of the magnetic field due to the magnetic dipole interactions can be neglected.[27] With the different magnitudes of the magnetic field, the Co nanoparticle will be redistributed, thus providing the platform with different arrangements. Probed molecules can locate themselves at highly SERS-active sites during the rearrangement of the metal nanoparticles. As is well known, the excitation of surface plasmons at smooth surface is usually not possible because of mismatch of the momentum of the photon with that of the surface plasmon.[35, 36] Thus, rough surface is necessary for the SERS measurement. Due to the superparamagnetic response of the Co nanoparticles, our prepared non-spherical Ag and Co SERS substrate simultaneously possesses both strong magnetic responsiveness and tunable plasmonic properties. Excellent SERS activity can be found in Fig.
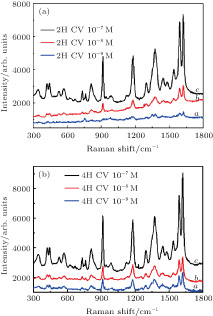
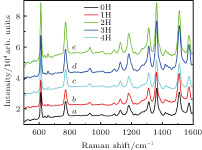
To explore the sensitivity of our prepared cobalt and silver SERS substrate, different concentrations of the investigated CV molecules are investigated, and the results are shown in Figs.
Employing 10−6-M R6G as the test molecules, we also investigate the SERS activity with and without magnetic field, and the results are shown in Fig.
In addition, we also carry out the SERS experiment with R6G probed molecule at 4H magnetic field to verify that our detection limit can be downloaded to 10−9 M. The results are shown in Fig.
The experimental results in Figs.
As a consequence of the surface plasmon resonance where the metallic nanoparticles provide the necessary enhancing properties and magnetic field responsive cobalt nanoparticles, the enhanced Raman effects can be obtained in the presence of the external magnetic field, with using our prepared non-spherical silver and cobalt SERS substrates. Here we pay special attention to the role of tunable magnetic field effect on the SERS activity, and the results show that the enhanced SERS effect can be obtained with a larger magnetic field. It happens due to the closely packed metallic nanoparticles under the influence of the external magnetic field. The local enhancement effects of test molecules (CV and R6G) are matched with each other. Thus, our experimental results illustrate that integration of cobalt and noble metal nanoparticles results in simultaneous magnetic activity and optical response. Employing such magneto–optical systems, it is a great desire to achieve dynamic control of localized surface plasmon resonance wavelength through external magnetic field, which would be a breakthrough in biology-related nanotechnology.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] |