† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant No. 51201181) and the Scientific Research Fund of Civil Aviation University of China (Grant No. 08QD14X).
First-principles calculations based on the density functional theory (DFT) and ultra-soft pseudopotential are employed to study the atomic configuration and charge density of impurity P in NiAl Σ5 grain boundary (GB). The negative segregation energy of a P atom proves that a P atom can easily segregate in the NiAl GB. The atomic configuration and formation energy of the P atom in the NiAl GB demonstrate that the P atom tends to occupy an interstitial site or substitute a Al atom depending on the Ni/Al atoms ratio. The P atom is preferable to staying in the Ni-rich environment in the NiAl GB forming P–Ni bonds. Both of the charge density and the deformation charge imply that a P atom is more likely to bond with Ni atoms rather than with Al atoms. The density of states further exhibits the interactions between P atom and Ni atom, and the orbital electrons of P, Ni and Al atoms all contribute to P–Ni bonds in the NiAl GB. It is worth noting that the P–Ni covalent bonds might embrittle the NiAl GB and weakens the plasticity of the NiAl intermetallics.
Nickel aluminum (NiAl) intermetallics exhibit a lot of attractive properties such as a high melting temperature, low density, good thermal conductivity, excellent corrosion, and oxidation resistance, making it have potential application in the aerospace industry as a new high temperature structure material.[1–3] Its practical application, however, is limited by poor ductility at low temperatures[3] and brittle grain-boundary fracture at elevated temperature.[4] There have been several attempts in the past to improve the mechanical properties of NiAl especially by micro-alloying with Mo, Ti, Ga, and Cr.[5, 6]
Many materials exist as polycrystals including NiAl,[3] so the defects of grain boundaries (GBs) are inevitable in the material. The GBs in metals and alloys offer favorable sites for the segregation of impurities. The segregation of impurity in GB can change the structure of GB, even cause a significant variation of the mechanical properties of the material.[7–10] Certain additives have been found to be desirable because they can enhance the cohesive properties of NiAl such as B and some alloying elements like Zr and Mo, while other impurities would cause a deleterious effect, O and C atoms for example, leading to the degradations of various properties of NiAl.[11–15] Thus, it is of great significance to study the NiAl GB to improve its mechanical properties.
In the polycrystalline NiAl intermetallics, a Σ5 GB has been suggested to have good crack resistance, thus causing it to have a strong boundary.[16] Also experimental studies of the Σ5 GB have proven that multiplicity in GBs structures can be observed in real metals. The meaning of the structural multiplicity of GBs structures is relevant to the crystal structure changes and mechanisms of defect interactions.[17]
Impurities such as P in NiAl play important roles in affecting the mechanical properties of this alloy. However, there are few reports about P alloying behavior in NiAl, especially for NiAl GB. For a long time, P was generally regarded as a deleterious element in superalloys and steels.[18, 19] A trace amount (ppm) of doping P principally segregates in the NiAl GB. The segregation of P in the NiAl GB hardens the GBs and hampers the process of dynamic recovery or recrystallization during its alloying deformation, leading to the formation of cavities in GBs, which influences the mechanical properties of NiAl.[20] Nevertheless, more and more advantageous effects of P on superalloys and steels have been observed.[21, 22] To better understand these effects, studies of the performance of P in NiAl are crucial.
In this article we employ first-principles calculations to study the site occupancy, atomic configuration, density of states and charge density of a P atom in the NiAl Σ5 grain boundary (GB), to try to understand the performance of P in the NiAl GB.
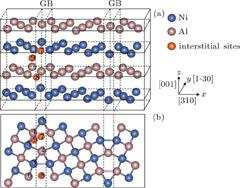
All calculations were based on the density functional theory (DFT) and ultra-soft pseudopotential as implemented in the Vienna Ab initio Simulation Package (VASP).[23, 24] We employed the generalized gradient approximation (GGA) with the Perdew and Wang (PW91).[25] 400 eV was used as a cutoff energy for the plane wave basis. The Brillouin zones were sampled with 2 × 4 × 8 k points by the Monkhorst–Pack scheme.[26] When a convergence criterion of the force on each atom was less than 10−3 eV/Å, all atomic positions were fully relaxed at a constant volume. The selected NiAl Σ5 (310)/[001] tilt grain boundary is considered to be a typical coincidence boundary in NiAl, which is formed by rotating a grain by 36.9° along the [001] axis and using (310) as its boundary plane. The supercell of NiAl Σ5 GB we constructed is shown in Fig.
For identifying the most preferred sites of P in the NiAl Σ5 GB, we first compute the formation energies in different cases after optimization of structure. We set up nine different and representative sites in the GBs for P atom (as shown in Fig.
The calculation formula of the formation energy is presented below. If the P atom substitutes for an Al or Ni atom, the formation energy reads
 | (2) |
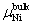
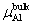
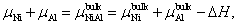 | (3) |
 | (4) |
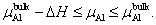 | (5) |
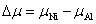
 | (6) |

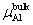
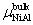
Figure
 , and the maximum is , and the maximum is  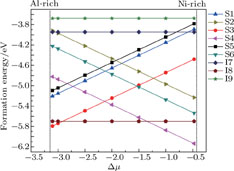 | Fig. 2. (color online) Variations of formation energy with Δμ for the NiAl GB occupied by P atoms at different sites. The minimum value of Δμ is   |
As shown in Fig.
Moreover, when the P atom occupies the interstitial site, the formation energies of I7 and I9 are much higher in the entire range of permissible chemical potential, while the formation energy of I8 is the lowest. After analyzing these three interstitial sites, we find that the P atom stays in the Al-rich environment at the sites of I7 and I9, while the site of I8 is in the Ni-rich environment, indicating that a P atom tends to occupy the site (I8) in the Ni-rich environment rather than the Al-rich environment in the interstitial cases.
In the most range of the permissible chemical potential except the extremely Ni-rich environment, the formation energy of the interstitial case of I8 is lower than those of the substitutional cases of S3 and S4. Thus, a P atom tends to occupy an interstitial site which is in the Ni-rich environment in NiAl GB for most of the range of the permissible chemical potential; in the extremely Ni-rich environment in the NiAl GB, a P atom prefers to substitute for an Al atom, which is the first nearest to the GB. So in the following sections, we choose S4 and I8 for further study.
We calculate the segregation energy of impurity P in the NiAl Σ5 GB. The impurity atom preferring to segregate into the GB has a negative segregation energy value, and the positive segregation energy value indicates that the impurity tends to stay in the bulk. The segregation energy ES of P in the NiAl GB can be computed by
 | (7) |
We choose the lower formation energy case of I8 in our calculation, and its segregation energy is −4.46 eV. So we can deduce that a P atom is preferable to segregate in the NiAl GB. The negative segregation energy of P impurity in the NiAl GB may be due to the fact that the GB gives more interspace for impurities than in the bulk, so a P atom prefers to stay in the NiAl GB rather than in the bulk. What is more, a P atom and Ni atoms might form new bonds which cause the negative segregation energy.
The McLean equation is used to estimate an equilibrium impurity segregation concentration.[34]
 | (8) |
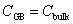
The typical temperature of 300 K–1500 K and a bulk P concentration of 500 appm–1000 appm are chosen.[35] Figure
Figure
 | Fig. 4. (color online) Atomic configurations of P atom and its first nearest Ni or Al atoms in the NiAl GB. |
In the interstitial cases, the ratio of Ni atom number in the first nearest-neighbor atoms to P atom number in the I8 case is higher than those of I7 and I9. In addition, figure
The S4 and I8 with lower formation energies are both in the Ni-rich environment, so we can deduce that there are interactions between a P atom and a Ni atom. It is possible that a P atom and a Ni atom form the P–Ni bond. We will make further research through charge distribution and density of states in the next two subsections.
Figures
As depicted in Figs.
 | Fig. 5. (color online) Charge distributions of a P atom with its first nearest atoms in the case of S4 and I8. The crystal faces for panels (a) and (c) are (001), (100) for panels (b) and (d). |
Hence, no matter whether a P atom is substituted with an Al atom or occupies the interstitial site, the contours of the P atom and the Ni atom overlap with each other, demonstrating that there can exist a possible interaction between a P atom and a Ni atom, and the formation of a Ni atom and a P–Ni bond might occur.
In order to better understand the interactions between a P atom and a Ni atom, we study the charge density difference. The deformation charge density Δρ should be calculated by
 | (9) |
Figure
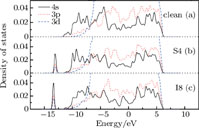
The density of states (DOS) can explain the bonding of P–Ni. We study two substitutional and interstitial cases whose formation energies are relatively low, i.e., S4 and I8. Figure
 | Fig. 7. (color online) Total densities of states for the clean, S4 and I8 cases, respectively, among which the Fermi energies are defined as being zero. |
To determine the roots of these small peaks appearing in the total DOS due to the addition of a P atom, we compute the local density of states (LDOS) of the P atom in the NiAl GB, i.e., S4 and I8. Figure
 | Fig. 8. (color online) Local densities of states of P atom in NiAl GBs for the (a) S4 and (b) I8 cases, in which the Fermi energies are both set to be zero. |
The LDOSs of Ni atom in NiAl GBs with P atom for S4 and I8 cases are shown in Fig.
The LDOSs of the Al atom in NiAl GBs with the P atom for S4 and I8 cases are shown in Fig.
First-principles calculations based on the density functional theory (DFT) and ultra-soft pseudopotential method are conducted to investigate the site occupancy, atomic configuration, density of states and charge distribution of the P atom in the NiAlΣ5 grain boundary (GB). The P atom whose segregation energy is −4.46 eV is demonstrated to prefer to segregate in the NiAl GB. The formation energy and atomic configuration of the P atom in the NiAl GB show that the P atom is preferable to stay in the Ni-rich environment in the NiAl GB forming P–Ni bonds. In most of the range of the permissible chemical potential, the P atom tends to occupy an interstitial site which is in the Ni-rich environment in the NiAl GB; when in the extremely Ni-rich environment in the NiAl GB, the P atom prefers to substitute for the Al atom which is the first nearest to GBs. As for charge density, the contours of P atom and Ni atom overlap with each other, indicating that a P atom likes to bond with a Ni atom rather than an Al atom. The deformation charge density also exhibits that a P atom and a Ni atom can form a new bond due to an obvious charge accumulation between the P atom and the surrounding Ni atoms. The observation of the density of states further demonstrates that there are possible interactions between a P atom and Ni atoms and the orbital electrons of P, Ni, and Al atoms all make contributions to P–Ni bonds in the NiAl GB. Significantly, the P–Ni covalent bonds might embrittle the NiAl GB and weaken the plasticity of the NiAl intermetallics. The theoretical results offer a meaningful reference for improving the mechanical properties of NiAl intermetallics.
| [1] | |
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] | |
| [11] | |
| [12] | |
| [13] | |
| [14] | |
| [15] | |
| [16] | |
| [17] | |
| [18] | |
| [19] | |
| [20] | |
| [21] | |
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] | |
| [27] | |
| [28] | |
| [29] | |
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] |