† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 51273133 and 51443004).
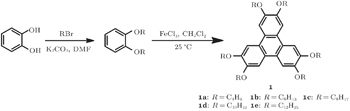
Disk-like liquid crystals (DLCs) can self-assemble to ordered columnar mesophases and are intriguing one-dimensional organic semiconductors with high charge carrier mobility. To improve their applicable property of mesomorphic temperature ranges, we exploit the binary mixtures of electronic donor-acceptor DLC materials. The electron-rich 2,3,6,7,10,11-hexakis(alkoxy)triphenylenes (C4, C6, C8, C10, C12) and an electron-deficient tetrapentyl triphenylene-2,3,6,10-tetracarboxylate have been prepared and their binary mixtures have been investigated. The mesomorphism of the 1:1 (molar ratio) mixtures has been characterized by polarizing optical microscopy (POM), differential scanning calorimetry (DSC), and small angel x-ray scattering (SAXS). The self-assembled monolayer structure of a discogen on a solid-liquid interface has been imaged by the high resolution scanning tunneling microscopy (STM). The match of peripheral chain length has important influence on the mesomorphism of the binary mixtures.
Miscibility of rod-like liquid crystals (LC) has been intensively studied because the blended homologous series of calamitic LC compounds have exhibited wider mesophase temperature ranges and lower melting points, which are very important for their commercial applications in LC displaying industry. Recently, an LC mixture containing a banana-shaped nematogen and a calamitic smectogen has reported to show nematic phase with lowered phase transition temperatures and widened mesophase ranges,[1] and a binary mixture of cross-like molecules exhibited biaxial nematic phase.[2] Discotic LCs are another kind of important mesomorphic materials: they self-assemble into columnar mesophases and have wide applications in display industry, field-effect transistors, organic light emitting diodes, and photovoltaic solar cells.[3–7]
To tune the supramolecular structures and the applicable properties, discotic LCs consisting of both electron-donor discogens and electron-acceptor discogens have been synthesized and investigated by our group.[8,9] However, the synthesis and purification of the discotic oligomers with covalent-bonded different discogens are not highly efficient. Therefore, we are interested in the blends of discotic LCs for applicable property improvement.
In the pioneering research work on discotic LC mixtures, Bushby and co-workers[10] have discovered that the complementary polytopic interaction (CPI) effect between triphenylene derivatives and two larger discotic systems greatly improves the mesomorphism. Park and coworkers[11] have reported electronic donor–acceptor pairs by blend electron-rich hexa(alkoxy)triphenylene with electron-accepting mellitic triimide to obtain much stable columnar mesophases. Mehl and coworkers[12] have reported a case of full miscibility of disk- and rod-shaped mesogens in nematic phase. Geerts and coworkers[13,14] have found that a phthalocyanine discotic mesogen and a perylenediimide lathlike mesogen are full miscible and the composites show a hexagonal columnar mesophase. Grelet and coworkers[15] have reported a case of two discotic columnar liquid crystals of perylene tetracarboxylate and a pyrene tetracarboxylate formed organic donor-acceptor heterojunction, which has potential application in photovoltaic solar cells. Song and coworkers[16] have shown that the miscibility of discotic truxene and truxenone derivatives. However, the investigation of binary mixtures of discotic mesogens is still in its infancy.
In this paper, we will report the phase behaviors of five binary mixtures of electron-rich 2,3,6,7,10,11-hexa(alkoxy)triphenylenes
A mixture of FeCl3 (13.01 g, 0.080 mol) in CH2Cl2 (20 mL) was cooled to 0 °C, 1,2-dibutyloxybenzene (5 g, in 20 mL CH2Cl2) was added slowly with stirring. The mixture was stirred 2 h at room temperature. Then, the mixture was cooled and cold methanol (15 mL) was added slowly. The solvent CH2Cl2 was distilled out under reduced pressure and the residue poured onto ice–water. The solid was collected by filtration, which was further purified by column chromatography (silica, eluted with CH2Cl2/petroleum 1:1) and recrystallization from ethanol to obtain
Equal mole of hexa(alkoxy)triphenylene
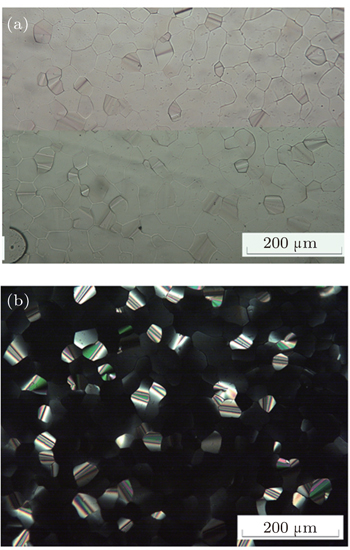
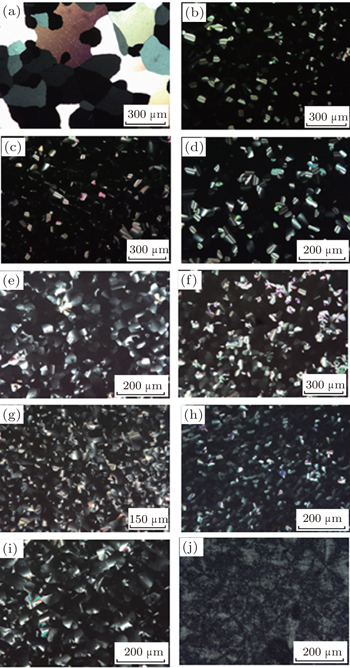
The mesomorphism of the 1:1 binary mixtures
It is noted that
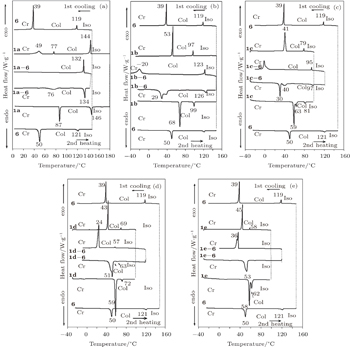
The phase transition temperatures have been measured by DSC, and the curves are drawn in Fig.
| Table 1. Thermodynamic properties of the discotic LC composites and the components (N2, heating and cooling rates 10 °C/min). . |
The DSC curve of
The composite
The mesophase of the blended composite has been further confirmed by small angel x-ray diffraction (SAXS) (Fig.
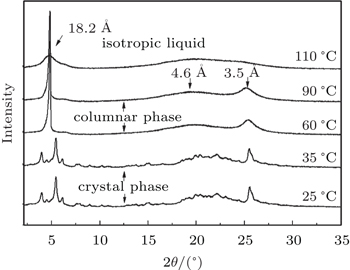 | Fig. 6. Small angel x-ray scattering (SAXS) patterns of the 1:1 binary mixture of composite |
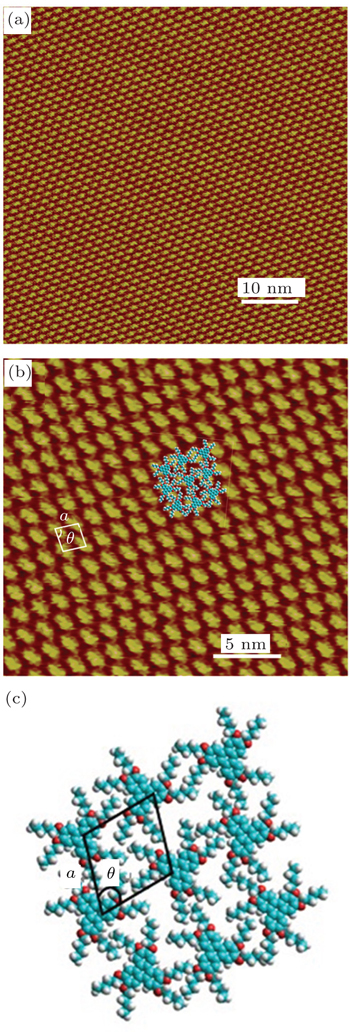
The self-assembled monolayer (SAM) structure of
The STM images of the blend
The binary mixtures of electron-rich organic semiconductors hexa(butoxy)triphenylene (
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 |