† Corresponding author. E-mail:
‡ Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 21402114 and 11544009), the Natural Science Basic Research Plan in Shaanxi Province of China (Grant No. 2016JM2010), and the Fundamental Research Funds for the Central Universities of China (Grant No. GK201603026).
Amphotericin B is a very effective antifungal drug, but it has an adverse reaction to the membrane of mammals’ cells. The interaction between AmB and cholesterol (Chol) causes the formation of pores on the membrane to destroy its integrity. In particular, AmB has a significant effect on the permeability of membrane for K+ ions. It has been reported that Na+ ions and Ca2+ ions may have some influence on the interaction between amphotericin B and lipid molecules. In this work, the effects of these metal cations on the physical state and intermolecular interaction of the Cholesterol/ Dipalmitoylphosphatidylcholine (Chol/DPPC) monolayer with and without AmB have been investigated. The addition of AmB induces the change of physical state of the lipid monolayer from liquid-gel phase to liquid phase. Different metal cations could influence the phase transition of the AmB-lipid monolayer. The K+ ions and Ca2+ ions make the obvious phase transition disappear. However, the presence of Na+ ions has little influence on the phase transition of the AmB-lipid monolayer. The addition of AmB and the presence of different metal cations weaken the attractive force on the monolayers. After addition of AmB, the force between the molecules is the strongest in the environment of K+ ions, thus is the weakest in the environment of Ca2+ ions, which may be due to the distribution of these metal cations inside and outside of cells. A large number of K+ ions distribute inside of the cells, thus most of Na+ and Ca2+ ions exist out of the cells. Hence, it may be possible that when AmB molecules are out of the cells, the reaction between the drug and lipid molecules is weaker than that inside the cells. These results may have a great reference value for further studying the toxicity mechanism of AmB and the influence of metal cations on the membrane.
Biological membrane is at the state of liquid crystal, on which the lipid composition mainly contains phospholipids and cholesterol. The normal physiological function of cell membrane depends on the fluidity of the membrane in liquid crystal.[1–5] By the manner of cell metabolism, organisms can constantly make the cell membrane play the best physiological function of its liquid crystal state.[6–9] If the state alters, it would be difficult for the biological membrane to maintain normal function.[10,11]
Amphotericin B (AmB) is a very effective antifungal drug with a heptaene macrolide skeleton,[12–15] at position C16 and C19 of which are respectively a functional carboxyl group and a mycosamine sugar appendage.[16] The antifungal activity of AmB is influenced not only by the phospholipid molecules of the membrane, but also by the sterols molecules largely.[17–21] AmB can aggregate on the membrane and form membrane pores with ergosterol molecules to cause fungal cell death.[22–25] However, the structures of cholesterol and ergosterol molecule are similar so that membrane pores are formed by interaction between AmB and cholesterol (Chol) on the host membrane, which produce toxicity to host cells.[26,27] The toxicity may be relative to the changes of liquid crystal state of membrane caused by it. AmB’s use in clinic is greatly limited because of its toxicity. Thus it is of great significance to study the toxicity mechanism of AmB and the influence of metal cations on the membrane.
At present, many of the researchers abroad and us have used the Langmuir technology and atomic force microscopy to study the influence of amphotericin B on the DPPC monolayer without Chol. However, the studies on the influence of metal cations on the AmB/Chol/DPPC monolayer were rarely reported. It is possible for some metal cations to be relative to the AmB’s cell toxicity. The pores in the membrane caused by AmB have an influence on physiological ion transport,[28,29] especially for K+ ions.[30] The drug could inhibit the ATP (Na+–K+) activity.[31] High sodium intake (> 4 mEQ/kg) per day may reduce the toxicity of AmB for premature infants.[32] K+ and Ca2+ ions have some effects on the aggregation of the drug.[33–36] Specifically, the addition of Chol can enrich the model membrane system and Chol may produce synergies with metal cations. Hence, it is of great importance to study the influences of K+, Na+, and Ca2+ ions on the interaction between amphotericin B and Chol/DPPC membrane.
In this work, we used the cholesterol / dipalmitoylphosphatidylcholine (Chol/DPPC) (3:7, mol/mol) monolayer as the model to simulate the liquid crystal biological cell membrane in half using Langmuir technology and atomic force microscopy. Different from previous ways, the data were fitted by the equation of state to obtain the parameters which can give some information on the intermolecular interaction. The characteristic of our work is that the influence of different metal cations on the interaction between AmB and Chol/DPPC monolayer were discussed according to the experiment data and the theoretical analysis. The influence of different metal cations on the AmB’s cell toxicity was discussed. The results are helpful for obtaining some information on the influence of different metal ions on the interaction between AmB and Chol/DPPC monolayer at the air–water interface.
Dipalmitoylphosphatidylcholine (DPPC: purity ≥ 99%), power amphotericin B (AmB: purity > 75%), cholesterol (Chol: purity ≥ 99%) and Tris(hydroxymethyl) aminomethane (Tris: purity ≥ 99.9%) were purchased from Sigma, USA. The other chemicals were of analytical grade and were used without further purification. DPPC and Chol (7:3, mol/mol) were dissolved in a chloroform/methanol mixture (9:1, v/v) to give a final concentration of 0.64 mM lipid membrane-forming solution. AmB (0.66 mM) was dissolved to a concentration of approximately 6 × 10−4 M in a 3:1 (v/v) mixture of dimethylformamide and 1 M HCl (aq). A subphase buffer composed of 10 mM Tris-HCl buffer, pH 7.4, was prepared for the Langmuir experiment. High-purity water was obtained from a Milli-Q plus water purification system (18.2 MΩ/cm, Millipore, USA). The concentrations of different metal cations were respectively 140 mM Na+, 5 mM K+, and 2.5 mM Ca2+ ions, and pH was 7.4.
The Langmuir monolayer is a most useful model membrane, which is employed in the research of the interaction of amphipathic molecules at the air–water interface in the perspective of physical chemistry.[37] It has mainly two unique features: 1) area per molecule can be controlled precisely; 2) simplicity of the physical model.
Langmuir monolayers are prepared by the Langmuir film balance. A Langmuir trough (KSV-Mini trough, Finland) was employed to study the monolayer behavior at the interface, and the surface pressure was measured with a Wilhelmy-type tensiometer using filter paper (10 mm × 30 mm × 0.15 mm). The accuracy of the sensor was 0.1 mN/m. Teflon barriers can symmetrically compress or expand the monolayer at a certain rate. For all the experiments, the temperature was maintained at 20 ± 1 °C.
Before each run, wash the Teflon trough (trough size 323 mm × 75 mm × 5 mm) with ethanol and rinse with purified water. The drug or lipid solutions are added at the air–water interface by a Hamilton microsyringe. Spread solvent evaporation over 10 min, then compress the monolayers at the speed of 75 cm2/min and obtain the surface pressure–area per molecule curves. Repeat the experiment three times.
Prepare Z-Langmuir film at a certain surface pressure and observe the structure of membrane by AFM (Shimadzu, Japan). In the contacting mode, the dipping rate for transfer was 5 mm/s. Use the silicon nitride pyramidal tip mounted on a 100-upmum-long cantilever with a force constant of 0.1 N/m.
Information on phase transition of lipid monolayer can be obtained by the surface pressure–area per molecule curve (π–A isotherm).[38] The elasticity modulus 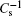
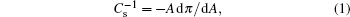
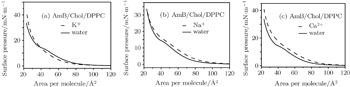
Whether or not these metal cations are in water, the π–A isotherms of the Chol/DPPC monolayers have no obvious inflection point, which suggests that the process of phase transition has not occurred (Fig.
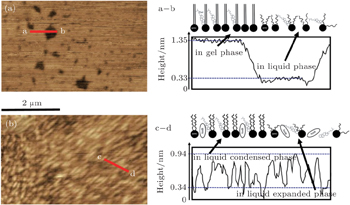
AmB can influence the physical phase of the Chol/DPPC monolayer, which also can be seen in AFM images of Fig.
After addition of K+, Na+, or Ca2+ ions into the subphase solution, the Chol/DPPC monolayer has a significantly different change in expansion of the α = 1 curve (Fig.
However, for the AmB/Chol/DPPC monolayer, its phase transition changes significantly after the addition of these metal cations (Fig. 
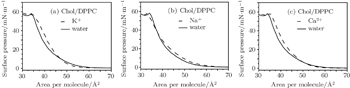 | Fig. 3. Surface pressure–area per molecule isotherms of the Chol/DPPC monolayer in the environment of different metal cations compared with that in water ((a) K+ ions, (b) Na+ ions, (c) Ca2+ ions). |
The information on the intermolecular interaction can be acquired from the ΔGexc of monolayer. It was calculated by the following equation for binary Langmuir monolayer:[42,43]

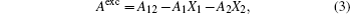
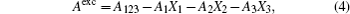
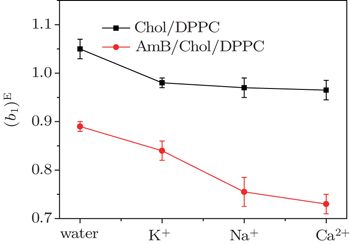
The ΔGexc values of the mixed monolayers are negative at 15 mN/m, which is close to the surface pressure of phase transition point. This supports that the interaction between the molecules is attraction. After addition of AmB, the absolute value of ΔGexc decreases, which indicates the weakening of the interaction on the monolayer. The phenomenon may be due to the fact that an AmB molecule combines with a Chol molecule to form the AmB-cholesterol complex,[44] which causes the formation of pore on the monolayer. Also in the environment of different metal cations, the attraction between the molecules in the two monolayer systems are also all weakened. The head group of AmB combines with the hydrophilic head of lipid by a van der Waals force. These head groups have a hydrophilic interaction with water molecules. After addition of the metal cations, they are combined with these head groups through a weak ionic bond, which is weaker than the hydrophilic interaction. The decrease of the van der Waals force between AmB and lipid molecules at the air–water interface may be due to the weak ionic bond.
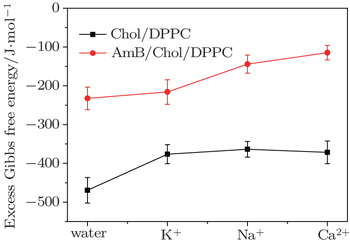 | Fig. 5. The ΔGexc of the monolayers at about the surface pressure of phase transition (15 mN/m) in water and in the environment of different metal cations. |
However, the strengths of the force between the head group and various metal cations have many differences. On the Chol/DPPC monolayer, the strength of intermolecular interaction in the environment of potassium ions is almost as strong as that in the presence of Na+ ions and Ca2+ ions. Significantly different, on the AmB/Chol/DPPC monolayer, the force is the strongest in the environment of K+ ions, thus is the weakest in the environment of calcium ions. Metal cations interact with −COO− group of AmB molecules. The interaction may be due to the weak ion pairs. The differences of these metal cations’ reactions may be due to their atomic scales. On the other hand, a large number of K+ ions distribute inside of cells thus most of the Na+ and Ca2+ ions exist outside of the cells. So when AmB molecules are outside of the cells, the reaction between the drug and lipid molecules is weaker than that inside the cells.
The simplest equation of state for the Langmuir monolayer is the two-dimensional ideal gas law (Eq. (
If the monolayer can be compressed to a finite area A0, the equation of state is given as follows:[47]
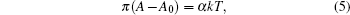

Thus, the following equation is the two-dimensional virial equation of state:[48]
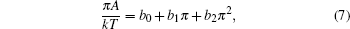
For a mixed monolayer, the second virial coefficient is as follows:[48]
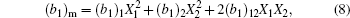
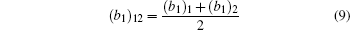
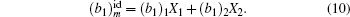
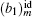
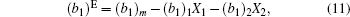
In Fig.
At the same surface pressure, these metal cations cause different changes in expansion of the π–A curve, which suggests that they could alter the area per molecule of the Chol/DPPC monolayer in liquid phase. In water, after addition of AmB, the area of each molecule of lipid monolayer decreases above a certain pressure thus increases below the certain pressure, which is the surface pressure at the point of transition of the AmB/Chol/DPPC monolayer from liquid expanded to liquid condensed phase. The addition of AmB induces the variation of physical phase of the lipid monolayer from liquid-gel phase to liquid phase. Different metal cations could mainly influence phase transition of AmB/Chol/DPPC monolayer. The K+ ions and Ca2+ ions make the phase transition disappear. However, the presence of Na+ ions has little effect on phase transition of the AmB-lipid monolayer. Addition of AmB and different metal cations weaken the attractive force on the monolayers. The degree of weakening is different in the environment of different metal ions. AmB could induce the phase transition and the ordering of the monolayer.
The orientation of the drug is relative to the interactions between the -COO- and -NH3+ groups and -OH groups of molecule. The -COO- group has stronger hydration than -NH3+, which means that the -COO- group has greater exposure to the liquid solution and orients AmB’s macrolide ring closer to the polar region. Different metal cations may interact specifically with the -COO- group by weak ion pairs. Chol may have a synergistic effect on the activity of AmB with different metal cations.
The difference of these metal cations affecting the role of AmB is associated with the mode of ions transportation in the membrane. The pores formed by Chol-AmB complex are permeable for potassium ions.[17] The K+ ions pass through the membrane into the cellular internal. However, the Na+ ions and Ca2+ ions cross the membrane to inside the cells by passive transport from high to low concentration. Differently, the charge number has a distinction in Na+ ions and Ca2+ ions. It is well known that Ca2+ ions have a special role in maintaining the stability of the membrane. After the treatment of AmB on human cells, AmB molecules combine with Chol on the membrane of cells to form pores, which may be affected by the transportation of these metal ions.
The influence of these metal cations on the AmB/Chol/DPPC monolayer is highly significant. The results in this work are helpful for further study of the toxicity mechanism of AmB and the influence of metal cations on the membrane. The metal cations not only relate to the membrane by the ion channel but also interact with the lipid molecule to influence the properties of membrane. We research the biological problem at the physical aspect of intermolecular interaction. However, the forms of AmB molecules have been unclear when the drugs interact with lipid molecules on the membrane of cells. The single molecule form and polymer form of AmB molecules may result in two manners of interactions. Also, do the two forms of AmB influence its toxicity of cells? These problems are of great importance for understanding the toxicity mechanism of AmB, which need further study.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 | |
| 39 | |
| 40 | |
| 41 | |
| 42 | |
| 43 | |
| 44 | |
| 45 | |
| 46 | |
| 47 | |
| 48 |