† Corresponding author. E-mail:
‡ Corresponding author. E-mail:
Project supported by the Fundamental Research Funds for the Central Universities, China (Grant No. 2014JBZ009) and the National Natural Science Foundation of China (Grant Nos. 61274063, 61377028, 61475014, and 61475017).
The sodium chloride methanol solution process is conducted on the conventional poly(3-hexylthiophene) (P3HT)/[6,6]-phenyl-C61-butyric acid methyl ester (PC61BM) polymer bulk heterojunction solar cells. The device exhibits a power conversion efficiency of up to 3.36%, 18% higher than that of the device without the solution process. The measurements of the active layer by x-ray photoelectron spectroscopy (XPS), atomic force microscopy (AFM), and ultraviolet photoelectron spectroscopy (UPS) indicate a slight phase separation in the vertical direction and a sodium chloride distributed island-like interface between the active layer and the cathode. The capacitance–voltage (C–V) and impedance spectroscopy measurements prove that the sodium chloride methanol process can reduce the electron injection barrier and improve the interfacial contact of polymer solar cells. Therefore, this one-step solution process not only optimizes the phase separation in the active layers but also forms a cathode buffer layer, which can enhance the generation, transport, and collection of photogenerated charge carriers in the device simultaneously. This work indicates that the inexpensive and non-toxic sodium chloride methanol solution process is an efficient one-step method for the low cost manufacturing of polymer solar cells.
Polymer solar cells (PSCs) are promising for low cost roll-to-roll manufacturing of flexible large area photovoltaic devices. To achieve high efficiency devices, the optimized morphology of the active layer for the improved generation and transport of charge carriers and the modified electrode for the facilitated charge extraction are essential.[1–3] The bulk heterojunction (BHJ) is more advantageous in the charge separation than the planar heterojunction due to the improved charge carrier kinetics by forming a morphological idea heterojunction network.[4,5] Thermal/solvent annealing can also enhance the phase separation of the active layer and help the charge separation.[6–8] Some reports indicated that the solvent additive can be used for morphological control in the polymer BHJ solar cells, and thus facilitate the charge separation and transport between the donor and acceptor in the active layer.[9,10] Previous studies have shown that the nanoscale domains of the active layer provide spatially isolated regions in which the holes and electrons can transport.[11] In addition, many groups have found that the device performance could be considerably enhanced by polar solvent treatment, which is thought to optimize the charge separation and transport in PSC active layers.[12–14] On the other hand, the interface engineering is vital to improving the performance of PSCs. Many experiments have reported that the performance of PSCs can be improved by inserting a cathode interfacial layer between the active layer and the cathode.[15] Various interfacial materials, such as metal oxides (e.g., ZnO, TiOx),[16–19] self-assembled monolayer,[20] lithium fluoride (LiF),[21,22] cesium carbonate (Cs2CO3),[23,24] cesium fluoride (CsF),[25] and sodium chloride (NaCl)[26] have been successfully used to enhance the performance of PSCs.
In this study, we demonstrate that the NaCl methanol solution treatment can well improve the device performance of PSCs due to the optimization of the phase separation in the active layer and the formation of a low work function surface in the cathode. The solution process is simpler than the traditional vacuum evaporation. The methanol solution process has some advantages over the deionized water method[26] in polymer solar cells. On one hand, the deionized water is believed to be a bad solvent for most organic materials and favors oxidation or reduction in the device under bias.[27] Especially, it has been reported that the deionized water has a degradation effect on the P3HT:PC61BM solar cells;[28,29] on the other hand, as a solvent, methanol can improve the morphology of the active layer, which is beneficial to the electronic collection and, as a result, improve the performance of the solar cells.[12,30,31] Our work demonstrates that the NaCl methanol solution process can help the charge separation and the generation of mobile carriers, transport of photogenerated electrons and holes, as well as the collection of electrons at the cathode. More importantly, this process can be used in the printing electronics manufacturing processes.
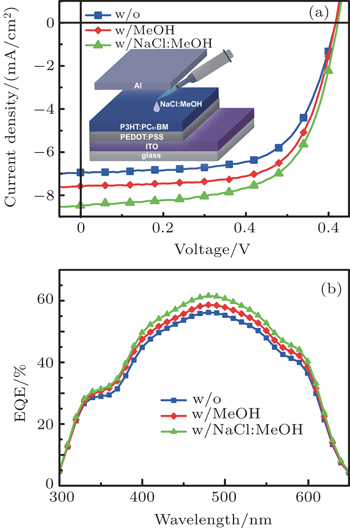
The device configuration of the PSCs is shown in the inset of Fig.
The devices were tested under one sun, AM 1.5 xenon-lamp-based solar simulator (San-Ei Electric) at 100 mW/cm2 intensity. Current density–voltage (J–V) characteristics of the devices were measured through a Keithley 6430 Source Measure Unit. The external quantum efficiency (EQE) measurements were carried out by using a solar cell QE/IPCE measurement system (Zolix solar cell scan100). The morphologies of the blend films were investigated by the Shimadzu Corporation SPM-9600 atomic force microscopy (AFM). X-ray photoelectron spectroscopy (XPS) and ultraviolet photoelectron spectroscopy (UPS) were performed using an ESCALAB 250 analysis system. Capacitance–voltage (C–V) measurements and impedance spectroscopy measurements were conducted with an impedance analyzer E4990A Agilent.
The device performances are exhibited in Fig.
| Table 1. Photovoltaic parameters of the PSCs with or without treatment based on over twenty devices. . |
The surface morphologies of the P3HT:PC61BM films are imaged by AFM, and the images of the control devices (without treatment and with the methanol treatment) and the NaCl methanol solution processed device are shown in Fig.
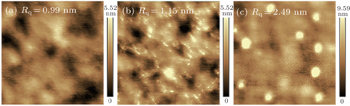 | Fig. 2. AFM (1 μm × 1 μm) images of P3HT:PC61BM blend films without treatment (a), with methanol (b), with methanol-processed NaCl (c). The root mean square roughness (Rq) is marked in each image. |
The surface compositions of the films are tested by XPS, and the results are shown in Table
| Table 2. Atomic fractions at the top surfaces of P3HT:PC61BM blend films with or without treatment. . |
The atomic fractions of C (1s), S (2p), Na (1s), and S (2p)/C (1s) atomic ratios at the top surfaces of the various films show that the S (2p) atomic fraction declines from 1.23% to 0.86% after the methanol treatment, indicating that PC61BM diffuses to the top surface. The methanol treatment can improve the distribution of the active layer and slightly enhance the vertical phase separation. Then, after the NaCl methanol solution treatment, the decline of S (2p) atom occurs, while the Na (1s) atom signal can also be detected with an atomic fraction of 0.14%. The P3HT:PC61BM weight ratios at the bottom and top surfaces can be evaluated by using S (2p)/C (1s) atomic ratios. The S (2p)/C (1s) atomic ratios for the devices with the methanol or NaCl methanol solution treatments are lower than the one without treatment. It should be pointed out that the device with methanol treatment has a little lower S (2p)/C (1s) atomic ratio than the device with NaCl methanol solution process, and it is possible that the NaCl crystal has a slight blocking effect. Anyway, these results imply that the methanol treatment is conductive to the vertical phase separation. And the crystal in Fig.
The various Al surface properties are investigated by ultraviolet photoelectron spectroscopy (UPS). The 50-nm-thick Al films are deposited on the ITO substrates and then modified with the methanol and NaCl methanol solution processes. Figure
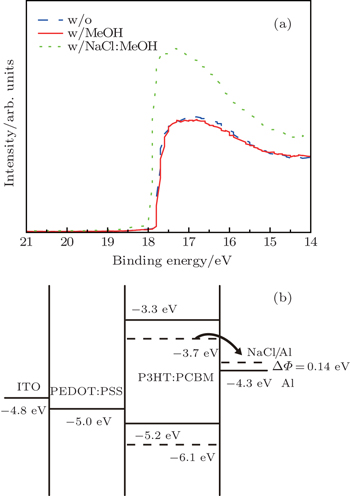 | Fig. 3. (a) UPS spectra of Al surface with and without treatment. (b) The band alignment of the component of the PSCs based on P3HT:PC61BM. |
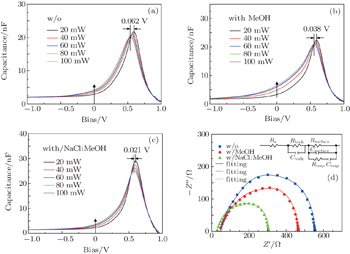
The C–V characteristics of the different devices shown in Figs.
The impedance spectrum is measured at a direct current (DC) forward bias of 0.62 V under dark conditions. Figure
We investigate the effect of the sodium chloride methanol process for bulk heterojunction organic solar cells. The XPS analysis of the active layer reveals a slight phase separation in the vertical direction and sodium chloride distributed island-like interface between the active layer and the cathode on the top surface. The C–V and impedance spectrum measurements prove that the sodium chloride methanol process can reduce the electron injection barrier and improve the interfacial contact of polymer solar cells. This work demonstrates that the sodium chloride methanol solution treatment can facilitate photoinduced charge separation, the generation of mobile carriers, and the collection of electrons.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 |