† Corresponding author. E-mail:
Project supported by the Natural Science Foundation of Inner Mongolia Autonomous Region, China (Grant No. 2013MS0809) and the Open Project of Key Laboratory of Functional Materials Physics and Chemistry (Jilin Normal University) of the Ministry of Education of China (Grant No. 201608).
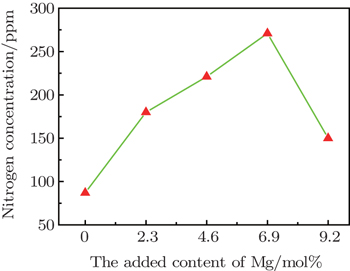
Diamond crystal crystallized in Fe–Mg–C system with Archimedes buoyancy as a driving force is established under high pressure and high temperature conditions. The experimental results indicate that the addition of the Mg element results in the nitrogen concentration increasing from 87 ppm to 271 ppm in the diamond structure. The occurrence of the {100} plane reveals that the surface character is remarkably changed due to the addition of Mg. Micro-Raman spectra indicate that the half width of full maximum is in a range of 3.01 cm−1–3.26 cm−1, implying an extremely good quality of diamond specimens in crystallization.
Diamond, as a typical traditional superhard material, can be synthesized by various techniques,[1–4] but the static-pressure-catalyst process is still the most popular way in the production of industrial diamond grits. In this method, the initial graphite and metallic catalyst experience high pressure and high temperature (HPHT) process either in a form of powder mixture or in a layer-by-layer assembly. The most effectively metallic solvent-catalysts used for diamond growth under the HPHT condition are prominently composed of Fe, Co, Ni, and Mn.[4–8] Besides, some oxygen-contained materials, such as Li2CO3, SrCO3, Na2CO3, and Na2SO4, can also serve as the catalytic medium for diamond growth.[9,10] Recently, the kimberlitic medium was selected as a catalytic solvent to explore diamond crystallization.[11] It is well recognized that the crystallized medium significantly affects the morphology and crystalline habit of diamond crystal. In recent years, more and more research works have focused on metals with a low melt point such as Mg, Cu, and Sn, acting as solvent metal for diamond growth.[12–14] The solubility of carbon source dissolved in these metal solvents is extremely low, and the required pressure for diamond crystallization is relatively high, at least ∼ 7 GPa, when a single element is used as the catalyst.[14] However, the spontaneously nucleated pressure for diamond crystals was reduced to ∼ 6 GPa in the Cu–Mg–C system.[12] In these systems, the dominant role of Mg is not clear enough. Accordingly, we explore the effects of Mg on diamond growth and properties in the Fe–Mg–C system under the HPHT condition in this paper.
As for diamond growth under the HPHT condition, the traditional methods are the phase conversion process, which is generally termed the film growth process, and the temperature gradient method. In the process of phase conversion, the driving force for the formation of diamond crystal is derived from the difference in thermodynamic stability between the two phases of carbon. In the temperature gradient growth process, the crystal growth can be controlled by the temperature gradient. In our work, a new growth process, in which carbon atoms diffuse from the carbon source to the crystallized sites through the metal melt with Arhchimedes buoyancy as a driving force, is used to control crystal growth.
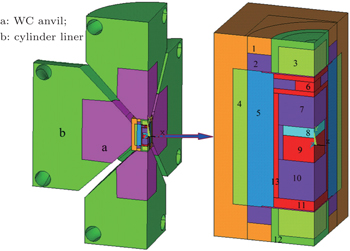
The experiments on diamond growth were performed in a cubic anvil HPHT apparatus (SPD-6 × 1400). The assembly for the synthesis of diamond crystals by a buoyancy process was designed as presented in Fig.
The high-purity graphite was utilized as a carbon source, and Fe1−xMgx (x value is in a range of 0–0.092) was used as the catalyst medium. The graphite powder (99.99% purity) was pressed into a cylindrical sample as the initial material loaded into the ampoule. After the HPHT synthesis with a run duration of 10 h, the collected crude samples were first treated with dilute nitric acid to separate the diamond grains from the metal catalyst medium. The diamond crystals were subsequently refluxed in the strong acid solution to eliminate the residual impurities present on the crystal surface. The as-synthesized diamond crystals free from visible inclusions were selected for analysis using an optical microscope. Nitrogen and hydrogen in these diamonds were characterized by micro-infrared (IR) spectra recorded from a Fourier transform infrared (FTIR) spectrometer in a spectral range of 500 cm−1–3500 cm−1 with a resolution of 2 cm−1 in a transmittance mode. For the IR measurements, a Bomem M110 FTIR spectrometer fitted with a microscope was employed. The IR beam size was rescaled to the range of 50 μm–150 μm in diameter for analyzing the absorptions only occurring in diamond crystal. Nitrogen concentrations (CN) in single substitutional form (C-form) were estimated by absorption coefficients of 1130 cm−1 (μ(130)) with a conversion factor of 25, namely, CN (ppm) = 25 × μ(1130) (cm−1). Morphologies and structural properties of the as-synthesized samples were characterized by scanning electron microscope (SEM). The crystalline quality of collected diamond samples was characterized by micro-Raman spectroscopy at room temperature.
The diamond crystallization is established in the Fe–Mg–C system under the HPHT condition by the buoyancy process. Typical SEM images from collected samples are shown in Fig.
 | Fig. 2. SEM images of specimens crystallized in buoyancy systems of the metals of Fe100−xMgx with x = 0 (a), 2.3 (b), 4.6 (c), 6.9 (d), and 9.2 (e), under the same HPHT condition. |
The role of Mg during crystal development is appreciably different from those of non-metal materials, such as S and P. As is well known, crystals generally have octahedral growth habits, when those nonmetallic materials are added in metal-solvent or serve as catalysts.[16,17] It should be pointed out that the degree of regularity of crystal turns worse and more macro-defects appear on the crystal surface as shown in Fig.
The IR detections of good-quality crystals which are collected from the Fe–Mg–C system indicate that nitrogen atom donors, even though without deliberate doping with nitrogen-related nitrides, are readily incorporated into diamond materials as a substitutional form, generally called C-center, characterized by 1130 cm−1 and 1344 cm−1 absorption peaks in one-phonon region as plotted in Fig.
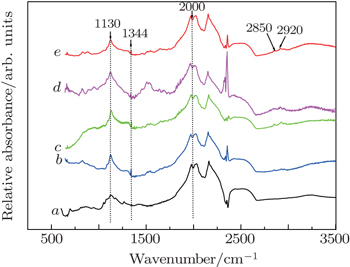 | Fig. 3. Typical IR spectra recorded for good-quality crystals grown by Fe100−xMgx, with x = 0 (curve a), 2.3 (curve b), 4.6 (curve c), 6.9 (curve d), and 9.2 (curve e). |
Evaluation on nitrogen content indicates that with increasing CMg in crystallization medium, CN progressively increases from 86 ppm to 271 ppm, before CMg < 6.9 mol%. The detailed variation of CN with the increasing of CMg added in solvent metal is presented in Fig.
The reason for causing the increase of CN while Mg is added into the crystallization medium is worth considering. Discussing the question on the possible role of the Mg element in catalysts, it is of interest to consider the role that the Ni element plays in Fe–Ni catalyst. As is well known, diamond crystal crystallized in pure Fe metal solvent generally has a lower CN, only at a level of tens of ppm. When Ni or Co is added into the catalyst/solvent, the CN in synthetic crystal will progressively increase up to around 200 ppm–300 ppm. The detailed CN in crystal depends on the crystallization temperature and the content of Ni and Co added in solvents. According to this experimental result, one can conclusively confirm that the role that the Mg element plays in the Fe–Mg catalyst system resembles the roles that Ni and Co metal play in the nitrogen incorporation process. Nevertheless, the addition of the Mg element does not enhance nitrogen aggregation like the Ni solvent. As reported, the synthetic diamond specimens are free from nitrogen in the Mg–C system, which is different from our result.[21] Considering the different conditions of diamond growth, for the Fe–Mg system we suggest that the Mg element plays a significant role in reducing the viscosity of solvent, leading to enhancement of the adsorption capacity in nitrogen impurity and therefore causing CN to rise in the crystal. As is well known, an appropriate content of Ni or Co added in pure Fe catalyst can reduce the crystallization condition. However, the experimental results in an Fe–Mg–C system indicate that the addition of Mg leads to the P–T range for diamond crystallization slightly moving upward.
The collected specimens are characterized by micro-Raman spectroscope in this study, and the results are displayed in Fig.
Diamond crystals readily crystallized in Fe–Mg–C system by using Archimedes buoyancy as a driving force. FTIR spectra indicate that the addition of Mg into the crystallization medium gives rise to an increase of CN in diamond structure, but higher crystallization temperature applied can depress the incorporation of nitrogen impurities into diamond structure. SEM observation demonstrates that diamond samples with high-quality surfaces are generally produced when 2.3 mol%–4.6 mol% of Mg is added in metal solvent. Micro-Raman spectra reveal that the crystalline quality of diamond specimens grown by this process is high, although some macro-defects occur in diamond specimens. It is found that the addition of Mg leads to the P–T range for diamond crystallization slightly moving upward, nevertheless {100} planes are present in crystals grown under the condition of CMg in a range of 6.9 mol%–9.2 mol%.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 |