† Corresponding author. E-mail:
Molecular dynamics simulation employing the embedded atom method potential is utilized to investigate nanoscale surface diffusion mechanisms of binary heterogeneous adatoms clusters at 300 K, 500 K, and 700 K. Surface diffusion of heterogeneous adatoms clusters can be vital for the binary island growth on the surface and can be useful for the formation of alloy-based thin film surface through atomic exchange process. The results of the diffusion process show that at 300 K, the diffusion of small adatoms clusters shows hopping, sliding, and shear motion; whereas for large adatoms clusters (hexamer and above), the diffusion is negligible. At 500 K, small adatoms clusters, i.e., dimer, show almost all possible diffusion mechanisms including the atomic exchange process; however no such exchange is observed for adatoms clusters greater than dimer. At 700 K, the exchange mechanism dominates for all types of clusters, where Zr adatoms show maximum tendency and Ag adatoms show minimum or no tendency toward the exchange process. Separation and recombination of one or more adatoms are also observed at 500 K and 700 K. The Ag adatoms also occupy pop-up positions over the adatoms clusters for short intervals. At 700 K, the vacancies are also generated in the vicinity of the adatoms cluster, vacancy formation, filling, and shifting can be observed from the results.
Surface diffusion and its related phenomena have been extensively studied in the last two decades and it is believed to be the most important controlling factor in most of surface dynamical phenomena, i.e., crystal growth, thin film growth, chemical reactions, and growth of island at the surface of a substrate.[1] One of the critical issues to uncover these processes is related to the diffusion behavior of the involved basic atomic groups including monomers and small atomic clusters.[2,3] For this purpose, a lot of works have been done on adatoms diffusion, which showed that the dynamics of adatoms are quite different and complicated.[4–6] Experimental studies of surface diffusion have been performed using a field ion microscope (FIM), which obtained valuable information about the diffusion.[7–10] However, direct visualization of the path of the adatoms, especially for the complex diffusion mechanisms, is still a bottleneck at the experimental side.
Molecular dynamics (MD) is one of the most suitable tools in relatively large scale simulation, which allows the tracking of motion of adatoms at finite temperature[11–19] that is not accessible from experiments and more advanced computational techniques such as first-principles calculations.[20,21] The results of MD simulation are found to be in good agreement with the experimental measurements with x-ray diffraction, FIM, and scanning tunneling microscope (STM).[22–24] The self-diffusion of Ag adatoms has been studied on Ag surfaces using various simulation techniques and the results revealed that hopping, jumping, and sliding are the common diffusion mechanisms, among which hopping is the dominant one on the (111) surface.[25–31] Diffusion of Cu (13-adatoms) on Cu (001) shows a significant difference in the diffusion behavior at different temperatures.[32] MD simulation of Cu 10-adatoms on Cu substrate revealed that dislocations and fissure are generated near the island on the surface of the substrate.[33] The higher stability and magic size effect for trimer and heptamer adatoms were also reported for the heterogeneous Ag/Cu system.[34,35] In recent years, extensive works have been done to study the hetero-diffusion of adatoms on (100) and (110) surfaces; the study showed that the hopping diffusion barrier is larger on the (100) surface.[36] Diffusion of Cu adatoms has been studied on Ag and Cu (111) surfaces using a theoretical approach. That work revealed that the dimer diffusion is difficult on the Cu surface compared to the Ag surface.[37] A first-principles study indicated that the surface diffusion of adatoms shows strong orientation dependence.[38]
Most of the works done so far are about the self-diffusion of metal; however very limited data are available for the heterogeneous surface diffusion process. Surface diffusion of heterogeneous adatoms cluster can be vital for the binary island growth on the surface. It can also be useful for the formation of alloy-based thin film surface through the atomic exchange process. This field has been chosen to understand the basic mechanism of heterogeneous adatoms diffusion on the metals as well as to understand the heterogeneity of adatoms in the cluster. For this purpose, the adatoms cluster is designed by mixing Cu and Zr atoms with suitable concentration.
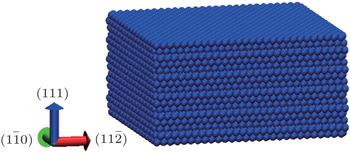
The configuration of the simulated substrate is shown in Fig. 
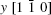
The highly optimized EAM potential is used to fit the potential energies of the surfaces (PES) of the systems derived from high-precision first-principles calculations performed using Vienna ab-initio simulation package (VASP).[39] The total energy Etot for the interaction can be calculated as
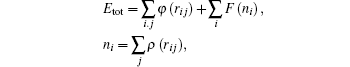
In constructing the PES, several properties of the elements are considered, including the mechanical properties, lattice dynamics, thermal behavior, energetics of materials, defects, deformation trajectories, and glassy structures. The EAM potentials have been tested against the experimental measurements for thermal expansion, melting behavior, and liquid dynamics via MD computer simulation.[40–43]
MD simulation code LAMMPS[44] is used to perform the simulation throughout this work. The bottom three layers of the Ag (111) substrate are kept fixed, the periodic boundary conditions are applied along the sides of the substrate, and the upper part of the substrate is subjected to the free boundary conditions. The substrate is first relaxed to minimum energy using the conjugate gradient method. The temperature of the system is kept constant using Nose Hoover thermostat and equations of motion are integrated using the velocity Verlet algorithm. A time step 1 fs is chosen for time integration of Newton’s equation of motion. The desired temperature is achieved by heating the substrate using NPT ensembles for 200 ps, followed by thermalization to achieve a uniform constant temperature. After proper thermalization, various adatoms clusters are placed over the substrate turn by turn and their motion is recorded and analyzed. The simulation process has been visualized and particular snapshots are taken using VMD.[45] The diffusion coefficient for the adatom island can be defined using equation
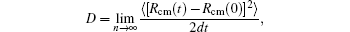
This section contains results of diffusion of the binary heterogeneous adatoms clusters of various sizes at three temperatures (i.e., 300 K, 500 K, and 700 K). We choose various sizes of binary adatoms clusters, including dimer (1Cu–1Zr), tetramer (2Cu–2Zr), hexamer (3Cu–3Zr), and octamer (4Cu–4Zr), to study various aspects of their diffusion. Finally, a brief discussion of the phenomenon of atomic exchange is presented.
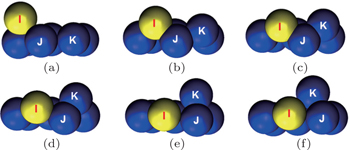
First of all, we construct a binary dimer consisting of 1Cu and 1Zr and place it at a randomly chosen position over the compact Ag (111) surface. The dimer movement at 300 K is simply hopping or jumping over different lattice sites, however the diffusion phenomenon is very complicated at higher temperatures, i.e., 500 K and 700 K. Some interesting snapshots of the diffusion of the dimer can be easily visualized by using VMD, as shown in Fig.
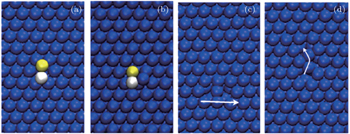 | Fig. 2. (a)–(d) Some typical snapshots of the heterogeneous dimer cluster. Blue, white, and yellow balls represent Ag (surface), Cu (adatoms), and Zr (adatoms), respectively. |
It is observed that the binary dimer adatoms can occupy different positions on the surface during the hopping process, i.e., the atoms may occupy either HCP or FCC site, or one atom occupies HCP site whereas the other is at FCC site and vice versa. The diffusion mechanism at 500 K is quite interesting as the hopping is much faster compared with that at 300 K. The Zr adatom exchanges with a surface Ag atom by knocking it out and becomes a surface atom, whereas the ejected Ag atom combines with the Cu adatom to form a (Cu–Ag) dimer adatoms cluster as can be seen from Fig.
At 700 K, exchange of Zr atom is more favorable due to the strong binding forces between the Zr and Ag atoms. The exchange leads to a new (Cu–Ag) dimer cluster which tends to separate soon and never recombine. The Cu atom also exchanges with the Ag atom by knocking it out from the surface and occupies the lattice site at the corner of the surface. Thermal vibrations near the adatoms also cause creation of vacancies at the surface by knocking out surface Ag atoms; the mechanism of vacancy shifting can be easily understood from the snapshots. Vacancies can propagate due to the shifting of one, two, or three atoms simultaneously.
The tetramer adatom cluster is composed of 2Cu and 2Zr; its diffusion over the Ag (111) surface is basically a sliding motion of a group of atoms between FCC and HCP sites with some shear motion as well as slight rotation at low temperatures. The diffusion mechanism of the tetramer can be understood by observing the selected snapshots in Fig.
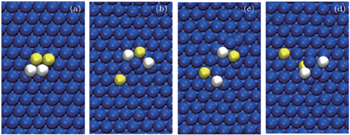 | Fig. 3. (a)–(d) Some typical snapshots of the heterogeneous tetramer cluster. Blue, white, and yellow balls represent Ag (surface), Cu (adatoms), and Zr (adatoms), respectively. |
Observations in MD for the tetramer diffusion reveal the simple hopping or jumping, shearing, sliding, and rotation at 300 K and 500 K, as can be seen from Fig.
For the hexamer cluster (contains 3Cu and 3Zr) on the Ag (111) surface, diffusion is very small at 300 K; however slight sliding, shearing, and rotational motions are more prominent at 500 K. The diffusion mechanism of haxamer can be explained with the help of snapshots taken at some critical points, which are shown in Fig.
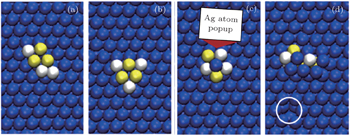 | Fig. 4. (a)–(d) Some typical snapshots of the hexamer cluster. Blue, white, and yellow balls represent Ag (surface), Cu (adatoms), and Zr (adatoms), respectively. |
It can be seen from Figs.
The motion of an octamer (4Cu and 4Zr) basically includes the concentrated motion, slight sliding and shear motion, and the diffusion of the binary octamer adatoms cluster is negligible for lower temperatures of 300 K and 500 K because of the higher potential energy barrier for the large size of adatoms cluster. The diffusion mechanism can be visualized from the chosen snapshots presented in Fig.
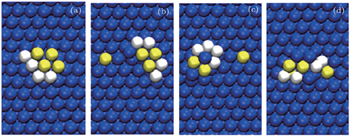 | Fig. 5. (a)–(d) The diffusion mechanism of the octamer cluster. Blue, white, and yellow balls represent Ag (surface), Cu (adatoms), and Zr (adatoms), respectively. |
To observe the trajectories of the adatoms cluster on the surface, the x and y coordinates of the center of mass are plotted in Fig.
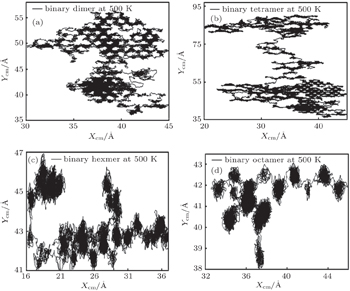 | Fig. 6. Traces of center of mass for heterogeneous adatoms clusters at 500 K: (a) dimer, (b) tetramer, (c) hexamer, (d) octamer. |
It can be seen from Fig.
To analyze the variation in the potential energy of the adatoms on the surface during their journey at the surface, the potential energy of the dimer adatoms is plotted against the simulation time and its values at 300 K, 500 K, and 700 K are compared as shown in Fig.
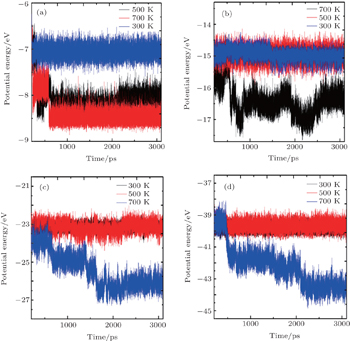 | Fig. 7. Potential energy of adatoms cluster versus simulation time plots for binary heterogeneous adatoms clusters at various temperatures: (a) dimer, (b) tetramer, (c) hexamer, (d) octamer. |
Figures
It is clear from Table
| Table 1. Calculated diffusion coefficients of heterogeneous adatoms clusters at various temperatures. . |
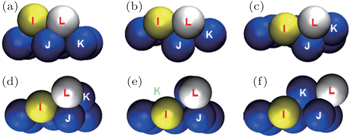
As discussed earlier, the adatoms placed over the Ag (111) surface may exchange and diffuse into the surface by knocking an atom out of the surface. The detailed analysis of the previous cases shows that this atomic exchange phenomenon depends mainly on three factors: temperature of the system (substrate & adatoms cluster), size of the adatoms cluster, and nature or type of the adatoms. To understand the mechanism of the exchange process, attention is focused on a single hexagon of surface over which a Zr adatom labeled ‘I’ is moved just before the exchange process as shown in Fig.
Figure
Molecular dynamics simulations with the EAM method are performed to investigate the diffusion process of adatoms cluster on Ag surface. The snapshots of the diffusion process, trace of center of mass, and potential energy of adatoms cluster are analyzed. At 300 K, the diffusion mechanisms of the adatoms clusters are simple hopping, sliding, and concentrated motion. At 500 K, small adatoms clusters (binary dimer) exhibit the atomic exchange process, where a Zr adatom exchanges itself with surface Ag atoms. No exchange of adatoms is observed for large size clusters (>dimer) and the movement is simply hopping, sliding, shearing, and concentrated motion over the substrate. At 700 K, the diffusion through the exchange mechanism is the most dominant phenomenon and preferably occurs for small adatoms clusters. Extra spaces and defects caused by thermal agitation at such a higher temperature enable the adatoms to penetrate into the surface by knocking out surface atoms. Small size adatoms clusters show higher probability for the exchange process due to weak bonding compared to those of large adatoms clusters. Hence the dimer exhibits the exchange process at 500 K as well as at 700 K, whereas the tetramer and large size clusters show exchange only at 700 K. The tendency of exchange of Zr atoms with the surface atoms is found to be much higher compared to that of Cu and Ag, where the probability of exchange of Ag adatoms is negligible.
The Zr adatoms prefer to stay close to each other in the form of a group, showing strong interaction between them (Zr–Zr) relative to other types of atoms. The strong tendency of Zr exchange is due to the strong interaction between Zr adatoms and Ag surface atoms. The Ag adatoms are frequently produced during the exchange process and tend to become a part of the adatoms cluster. Consequently, it shows no special activity, however it sometimes stays at the pop-up position over the adatoms clusters for short time intervals. Separation and recombination of one or more atoms from the group are also observed several times in almost every case at 500 K and 700 K. The vacancies are also created at 700 K near the adatoms cluster or near the edges of the simulation box, which may move by shifting their positions from one point to the other. The vacancies are also observed to be filled by some adatoms. The vacancy formation process is more prominent for the small adatoms clusters on the surface; however it may form near any cluster (large or small).
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 | |
| 39 | |
| 40 | |
| 41 | |
| 42 | |
| 43 | |
| 44 | |
| 45 | |
| 46 |