† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant No. 51276016).
The research of magnetic separation starts from magnetic solid particles to nanoparticles, and in the research progress, particles become smaller gradually with the development of application of magnetic separation technology. Nevertheless, little experimental study of magnetic separation of molecules and ions under continuous flowing conditions has been reported. In this work, we designed a magnetic device and a “layered” flow channel to study the magnetic separation at the ionic level in continuous flowing solution. A segregation model was built to discuss the segregation behavior as well as the factors that may affect the separation. The magnetic force was proved to be the driving force which plays an indispensable role leading to the segregation and separation. The flow velocity has an effect on the segregation behavior of magnetic ions, which determines the separation result. On the other hand, the optimum flow velocity which makes maximum separation is related to the initial concentration of solution.
Magnetic separation technology has been a hot area of research with its advantages of low cost, low energy consumption, high efficiency, ease of use, and so on.[1,2] In recent years, magnetic separation has made great progress and has a broad application prospect in many fields. It can be used to separate target samples mixed with magnetic nanoparticles, as the pretreatment of many analytes such as biological macromolecules, cells, antibodies, organic compounds, and so on.[3–6] The high gradient magnetic separation technology is proved to be useful in the removal of magnetic particles and lubricant pollution in sticky oil.[7] Magnetic separation technology can be applied to the environment when using magnetic nanoparticles as carriers to remove heavy metal ions or organic dyes in aqueous solutions.[8–10] Besides, by studying the feasibility of removing algae in fish ponds and the separation of binary mixtures of sorbitol and sucrose, it is proved that magnetic separation also has applications in ecology and food processing.[11,12]
In recent years, the research starts from the magnetic solid particles[13] to the magnetic nanoparticles,[14–18] and in the research progress, particles become smaller gradually with the improvement of research and applications. Nevertheless, few experimental studies of magnetic separation of molecules and ions under continuous flowing conditions have been reported.[19–24] Currently, the research objects of magnetic separation are mainly superparamagnetic nanoparticles represented by Fe3O4. These magnetic particles usually have large magnetic susceptibility and size, which makes the magnetic force large enough under the gradient magnetic field of thousands of Gausses (G). Compared with magnetic particles, the magnetic moment of molecules and ions is much smaller (for example, the magnetic susceptibility of a ferric chloride molecule is about 12 orders of magnitude smaller than that of a Fe3O4 particle). Thus, the magnetic force of molecules or ions is very weak under the same conditions. Besides, molecules or ions are very different from nanoparticles, and there are many obstacles such as a variety of interactions in the separation process. It is very difficult to achieve the magnetic separation of molecules and ions by common methods.
The separation of molecules or ions is needed in many potential applications with the development of science and technology. In the rectification column, for example, magnetic separation technology can be used as an assistant for the separation of liquid oxygen and liquid nitrogen. In the applications of industry, how to improve the recovery rate and reduce the energy consumption of the rectification column has been a common concern. As the temperature and pressure of the rectification column are difficult to control, complete separation is impossible. The residual liquid flowing from the bottom of the rectification column often leads to a waste of energy. However, the target liquid in the residual liquid can be further separated and enriched by using magnetic separation technology. When putting the residual liquid into the rectification column for cyclic utilization, the recovery rate will be improved. Correspondingly, the waste of energy is avoided. Moreover, flow behavior is always the key point in the research of magnetic separation technology. Therefore, it is a challenging and important scientific issue to research the magnetic separation of molecules and ions.
In this work, the ferric chloride (FeCl3) solution was used to study the possibility of magnetic separation at the ionic level in a homogeneous mixed solution. FeCl3 molecules are paramagnetic, and the magnetic susceptibility[25] is χm = 13450 × 10−6 cm3 · mol−1. FeCl3 molecules mainly exist in the form of Fe3+, Cl−, and hydrated ions like [Fe(H2O)6]3+ in an aqueous solution.[26] The magnetic susceptibility of molecules or ions is much smaller than that of magnetic nanoparticles, thus the magnetic force is very weak in a normal strength gradient magnetic field. Besides, many hindering factors such as diffusion exist in the separation, and the movement and diffusion of ions are greatly influenced by the flow state. Therefore, it is hard to confirm whether the magnetic separation of FeCl3 solution can be achieved. In this research, a magnetic field device with large magnetic field gradient and a “layered” flow channel that can easily achieve laminar flow were designed to study the possibility of the magnetic separation of FeCl3 solution. This study also built a segregation model to discuss the segregation behavior as well as the factors that may affect the magnetic separation, which could provide theoretical and experimental basis for magnetic separation at the molecular and ionic level in homogeneous mixed solution.
The magnetic field device is shown in Fig.
The structure of the flow channel is shown in Fig.
 | Fig. 2. Schematic diagram of flow channel structure and magnetic separation. x: channel width, y: channel length, l: channel height. |
Besides the magnetic device and the flow channel, a DHL-A constant flow pump with a steady flow device is used to provide power to the flow of the fluid, and a WGD-8A grating spectrometer is used to measure the concentration of the solution.
When FeCl3 solution is flowing in a gradient magnetic field, magnetic force, Lorentz force, Coulomb force, and various kinds of fluid resistance will have an effect, of which magnetic force only acts on Fe(III) ions (to facilitate the presentation, Fe(III) is used as the representative of paramagnetic ions such as Fe3+ and its hydrated ions). It has been shown that a large group composed of metal ions and water molecules moves in a magnetic field by magnetic force.[27,28] The magnetic force can be expressed[29,30] as 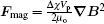
The flow behavior of the magnetic separation of FeCl3 solution is described in the form of the convection–diffusion equation under the effect of magnetic field
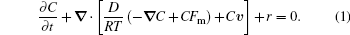

It is not hard to see that the magnetic force, the flow velocity and the concentration are the factors that influence the magnetic separation of FeCl3 solution. The influence of the magnetic force is obvious, the greater the magnetic force is, the better the separation will be. But it is difficult to read directly from the equation how the flow velocity and concentration affects the separation, therefore, experimental research is needed.
It is difficult to observe and measure the movement of Fe(III) ions directly in the magnetic separation at the ionic level. Therefore, the concentration and concentration difference were measured as a breach to study the separation.
The FeCl3 solutions with mass fraction about 13% were used, and the inlet flow rate was 2 ml/min. Then the concentrations in the two outlets were measured and compared with each other when the flow channel was placed in area I and area II, respectively. This was used to verify whether the magnetic force is the major driving force in the magnetic separation of FeCl3 solution. When the flow channel is located in area I and area II, respectively, the directions of the magnetic force of Fe(III) ions are just the opposite, but the directions of the Lorentz force are the same. Therefore, if the magnetic force is the major driving force, the results of the concentration in the two outlets are certainly the opposite when the flow channel is located in area I and area II, respectively.
Then the flow channel was placed in area I, and FeCl3 solutions of 15%, 17%, and 20% (mass fraction) were used, respectively. The concentration difference between the two outlets was measured when the flow rate was 1 ml/min, 2 ml/min, 3 ml/min, 4 ml/min, 6 ml/min, and 8 ml/min, respectively (the corresponding flow velocity is about 0.33 mm/s, 0.67 mm/s, 1.0 mm/s, 1.3 mm/s, 2.0 mm/s, and 2.7 mm/s, respectively). The influence of the flow velocity and the concentration on the magnetic separation of FeCl3 solution were studied by the change of the outlet concentration difference versus the flow velocity and the concentration.
The concentrations in the two outlets when the flow channel is located in area I and area II respectively are shown in Fig.
 | Fig. 3. The concentrations in the two outlets when the flow channel is located in different areas, with the initial concentration of the solution of (a) 12.6% and (b) 13.2%. |
The results of the concentration difference between the two outlets versus different flow velocity when the flow channel is located in area I are shown in Fig.
The enrichment of Fe(III) ions leads to the increase of the chemical potential. The chemical potential can be expressed[31] as 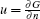


The magnetic field gradient is far greater at the exit position than that in the channel because of the sudden disappearance of the magnetic field at the exit of the channel. The Fe(III) ions are intercepted by greater magnetic force at the exit position, and only a few Fe(III) ions can flow out. Therefore, a certain accumulation of Fe(III) ions is formed (accumulation area in Fig.
The volume of the two equal areas in the flow channel is expressed by V0, then the concentration of Fe(III) ions in area 1 and area 2 can be expressed respectively as


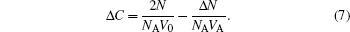
According to Eq. (

When Fe(III) ions are accumulated in the accumulation area, the viscous resistance pointing to the outlet will have an effect. The accumulation of Fe(III) ions also leads to the change of the chemical potential, which causes Fe(III) ions to be affected by the chemical potential driving force pointing to the outlet. At the steady state, the accumulated Fe(III) ions are balanced by the intercepted magnetic force, the chemical potential driving force and the viscous resistance at the exit position

According to Eq. (
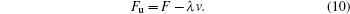
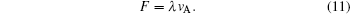
According to Eq. (
It has been shown that the flow velocity vA which makes ΔN = 0 is constant, and the change of ΔN with the flow velocity v is determined by the constants F and λ. Therefore, the optimum flow velocity which makes the maximum separation be dependent on the critical flow velocity v0, according to Fig.
The optimum flow velocity of 15%, 17%, and 20% FeCl3 solution are shown in Fig.
It has been shown that the optimum flow velocity is dependent on the critical flow velocity v0. When increasing the initial concentration of the solution, v0 is increased. Therefore, the optimum flow velocity is also increased.
The magnetic separation of magnetic ions has been achieved by the magnetic field device with larger magnetic field and gradient as well as the flow channel that can easily achieve laminar flow. The magnetic force is the major driving force, which plays an indispensable role, leading to the segregation of magnetic ions. The maximum number of the segregated ions is determined by the joint action of the chemical potential and magnetic force. The flow velocity also has an effect on the segregation behavior. When the flow velocity increases to a certain degree, the number of the segregated ions in the channel will start to reduce. As a consequence of the interception at the exit position of the channel, segregated ions accumulate. The number of the accumulated ions will be decreased when the flow velocity increases. As a result, the separation effect increases first and then decreases with the increase of the flow velocity. There is an optimum flow velocity which makes maximum separation. In this experiment, the optimum flow velocity is related to the initial concentration of solution, the higher the concentration, the larger the optimum flow velocity.
This study has some guiding significance for the magnetic separation of liquid oxygen and liquid nitrogen. It indicates that magnetic force plays a decisive role in the maximum separation effect when the flow velocity is optimum. Improving the performance of the magnetic device, such as using superconducting magnets, can greatly improve the magnetic force. Therefore, the separation effect will be improved to a certain extent. When using magnetic separation technology as an assistant for the separation of liquid oxygen and liquid nitrogen in the rectification column, the liquid oxygen in the residual liquid will be enriched greatly. As a consequence, the recovery rate will be improved when putting the residual liquid into the rectification column for cyclic utilization. Correspondingly, the energy is also saved. Besides, the equipment of magnetic separation technology is reusable, and no more energy consumption exists. Therefore, magnetic separation technology has great potential application in the separation of liquid oxygen and liquid nitrogen in the rectification column.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 |







