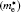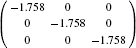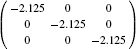† Corresponding author. E-mail:
Project supported by the National Basic Research Program of China (Grant No. 2014CB643703), the National Natural Science Foundation of China (Grant Nos. 11164005, 11464008, and 51401060), the Natural Science Foundation of Guangxi Zhuang Autonomous Region, China (Grant Nos. 2014GXNSFGA118001 and 2012GXNSFGA060002), and the Fund from Guangxi Provincial Key Laboratory of Information Materials of Guangxi Zhuang Autonomous Region, China (Grant Nos. 1210908-215-Z and 131022-Z).
The detailed theoretical studies of electronic, optical, and mechanical properties of γ-Bi2Sn2O7 are carried out by using first-principle density functional theory calculations. Our calculated results indicate that γ-Bi2Sn2O7 is the p-type semiconductor with an indirect band gap of about 2.72 eV. The flat electronic bands close to the valence band maximum are mainly composed of Bi-6s and O-2p states and play a key role in determining the electrical properties of γ-Bi2Sn2O7. The calculated complex dielectric function and macroscopic optical constants including refractive index, extinction coefficient, absorption coefficients, reflectivity, and electron energy-loss function show that γ-Bi2Sn2O7 is an excellent light absorbing material. The analysis on mechanical properties shows that γ-Bi2Sn2O7 is mechanically stable and highly isotropic.
Metal oxides with the pyrochlore structure and adopting a general formula A2B2O7 have received considerable attention due to their wide technological applications in catalysis,[1] gas sensors,[2] frustrated magnetism,[3] and superconductivity.[4] As one of the pyrochlore-type oxides, bismuth pyrostannate (Bi2Sn2O7) has been widely studied as one of the versatile and technologically important semiconducting materials with a wide band gap. Bi2Sn2O7 was first reported by Roth[5] and lately characterized by Brisse and Knop.[6] Previous experimental investigations have confirmed that Bi2Sn2O7 has three polymorphs. At room temperature, Bi2Sn2O7 probably adopts a monoclinic structure and is designated as the α phase.[7] At intermediate temperature higher than 90 °C, a cubic acentric phase (β phase) is found. At temperature above 680 °C, Bi2Sn2O7 is the ideal pyrochlore structure with cubic symmetry and designated as the γ phase.[8]
Being similar to other Bi-containing compounds like BiFeO3,[9] Bi2WO6,[10] and BiVO4,[11] the photocatalytic property of Bi2Sn2O7 has also been investigated extensively, owing to its potential application in the splitting of water or degradation of organic pollutants under visible light irradiation.[12–14] Wu et al.[12] prepared the nanocrystalline γ-Bi2Sn2O7 by a hydrothermal route and evaluated the photocatalytic activity by the degradation of methyl orange. Tian et al.[13] reported the synthesis of Bi2Sn2O7 and visible-light photocatalytic performance in the oxidation of As(III). Xu et al.[14] studied the fabrication and photocatalytic activity of BiOI/Bi2Sn2O7 heteronanostructure. On the theoretical side, Walsh and Watson[15] calculated the electronic structures of both α- and γ-Bi2Sn2O7 and highlighted the importance of covalent interactions between the electronic states of the metal with O-2p in Bi2Sn2O7. Very recently, Fan et al.[16] investigated the electronic structures of Bi2−xYxSn2O7 solid solution by performing density functional theory (DFT) calculations. In the present work, we devote great effort to investigating the optical and mechanical properties of γ-Bi2Sn2O7 besides its electronic structure by using the first-principles DFT calculations in order to provide some helpful guidelines for the subsequent experimental work.
All DFT calculations are performed by the projector augmented wave (PAW) method[17] as implemented in the Vienna ab-initio simulation package (VASP).[18,19] The exchange-correlation energy is treated within the generalized gradient approximation, using the functional of Perdew, Burk, and Ernzerhof.[20] The plane-wave energy cutoff is set to be 400 eV. A k-point spacing of about 2π × 0.03 Å−1 is used to generate the Monkhorst-Pack grid[21] in the Brillouin zone sampling. The fully relaxed optimization is carried out until the changes in total energy and the force on each atom are less than 10−5 eV and 10−3 eV/Å, respectively.
For optical properties calculations, a sufficient dense k-point grid spacing of 2π × 0.015 Å−1 is used. The dielectric function ε (ω) = ε1(ω) + iε2(ω) fully describes the optical properties of materials at all photon frequencies. The imaginary part ε2(ω) of dielectric function can be derived from the electronic band structure by summing all allowed direct transitions from the occupied to the unoccupied states.
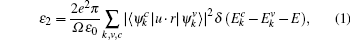
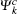
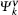
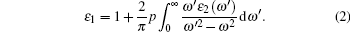
The pyrochlore-type Bi2Sn2O7 shown in Fig.
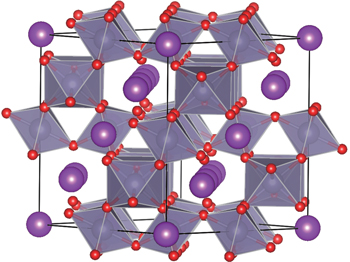 | Fig. 1. Crystal structure of the pyrochlore-type γ-Bi2Sn2O7. The octahedrons sharing vertices represent SnO6 subunits, where Sn atoms are located in the center and O atoms at the vertices. |
| Table 1. Optimized structural parameters of γ-Bi2Sn2O7. The experimental values[24] are given in the following parentheses for comparison. . |
The calculated electronic band structure of γ-Bi2Sn2O7 is plotted in Fig. 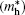
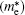
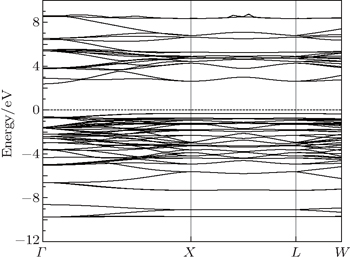 | Fig. 2. Calculated electronic band structure of γ-Bi2Sn2O7 along the selected high symmetry lines. The Fermi level is set to be zero for reference. |
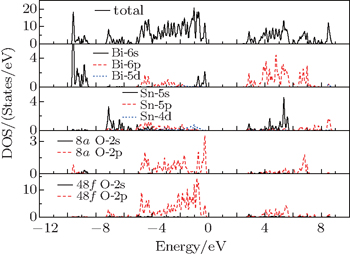
Figure
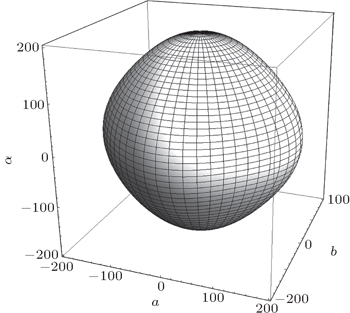
| Table 2. Calculated effective mass tensors of electrons |
Figure
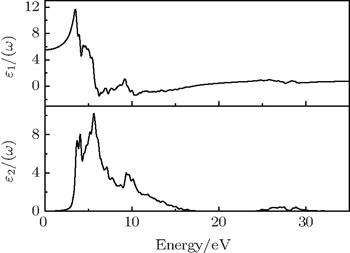 | Fig. 4. Calculated dependences of real part ε1(ω) and imaginary part ε2(ω) of the dielectric function of γ-Bi2Sn2O7 alloys on photon energy. |
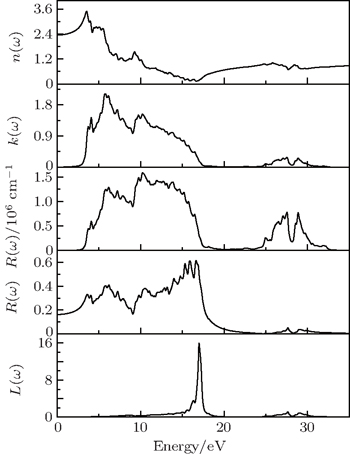
Based on the obtained dielectric function, the refractive index n(ω), extinction coefficient k(ω), absorption coefficients α(ω), reflectivity R(ω), and electron energy-loss function L(ω) are further calculated to check the optical properties of γ-Bi2Sn2O7 (see Fig.
The calculated elastic constants Cij of γ-Bi2Sn2O7 are listed in Table
| Table 3. Calculated values of elastic constant Cij, bulk modulus B, shear modulus G, Young’s modulus E, and Poisson’s ratio υ of γ-Bi2Sn2O7. . |
The bulk modulus B and shear modulus G are obtained by Voigt–Reuss–Hill approximation.[25] Young’s modulus E and Poisson’s ratio υ can be further calculated according to their relationships with B and G. The corresponding mechanical stability criterion for cubic crystal is that B, C11–C12, and C44 are positive.[26] It can be found from Table
The electronic, optical, and mechanical properties of γ-Bi2Sn2O7 are investigated by using the first-principles DFT calculations. Our results show that γ-Bi2Sn2O7 is the p-type semiconductor with an indirect band gap. The calculated band gap is about 2.72 eV and in excellent agreement with the experimental result. The hybridization interaction between Bi-6s and O-2p states in the valence bands close to the Fermi level is expected to play an essential role in determining the electrical behavior of γ-Bi2Sn2O7. The dielectric function and macroscopic optical constants including refractive index, extinction coefficient, absorption coefficients, reflectivity, and electron energy-loss function are also calculated to check the optical properties of γ-Bi2Sn2O7. Especially, the average reflectivity is about 30%, which indicates that γ-Bi2Sn2O7 is a good light absorbing material used for optical semiconductor devices and photocatalysts. Moreover, γ-Bi2Sn2O7 is mechanically stable and ductile according to the calculated elastic constants, bulk modulus, Young’s modulus, and Poisson’s ratio. The further calculations of the directional dependence of Young’s modulus show that γ-Bi2Sn2O7 is highly isotropic.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 |