† Corresponding author. E-mail:
Project supported by the Natural Science Foundation of Jiangxi Province, China (Grant Nos. 20152ACB21014, 20151BAB202006, and 20142BAB212002) and the Fund from the Jiangxi Provincial Educational Committee, China (Grant No. GJJ14254). Bo Xu is also supported by the Oversea Returned Project from the Ministry of Education, China.
The adsorption and diffusion behaviors of alkali and alkaline-earth metal atoms on silicane and silicene are both investigated by using a first-principles method within the frame of density functional theory. Silicane is staler against the metal adatoms than silicene. Hydrogenation makes the adsorption energies of various metal atoms considered in our calculations on silicane significantly lower than those on silicene. Similar diffusion energy barriers of alkali metal atoms on silicane and silicene could be observed. However, the diffusion energy barriers of alkali-earth metal atoms on silicane are essentially lower than those on silicene due to the small structural distortion and weak interaction between metal atoms and silicane substrate. Combining the adsorption energy with the diffusion energy barriers, it is found that the clustering would occur when depositing metal atoms on perfect hydrogenated silicene with relative high coverage. In order to avoid forming a metal cluster, we need to remove the hydrogen atoms from the silicane substrate to achieve the defective silicane. Our results are helpful for understanding the interaction between metal atoms and silicene-based two-dimensional materials.
Since the discovery of graphene, two-dimensional (2D) materials have attracted a great deal of attention by the research community due to the superiority of their excellent physical and chemical properties utilized in next generation nano-electronic and nano-optoelectronic devices.[1–4] Nevertheless, the incompatibility with the current silicon-based electronic technology hinders the potential application of the graphene. Silicene, a silicon analogue of graphene, has also attracted increasing attention for its novel chemical and physical properties,[5] such as the electric response,[6,7] ferromagnetism,[8,9] half-metallicity,[10,11] quantum Hall effect,[12] giant magnetoresistance,[13] and superconductivity.[14] Unlike planar graphene, the stablest silicene prefers a low-buckled (LB) structure, which is attributed to the mixing of its sp2/sp3 hybridized orbitals. The electronic structure of silicene is quite similar to that of graphene, exhibiting a semi-metallic character, in which the electrons at the Fermi level behave like massless Dirac fermions, thus leading to an extremely high carrier velocity.[15] As a consequence, the nano-devices fabricated by silicene will be more conveniently incorporated into the current silicon-based microelectronic industry without using carbon instead of silicon.
Adsorbing foreign atoms on silicene has significant potential applications. The adsorption of metal atoms provides a route to modifying the electronic properties of silicene. Ni et al. theoretically predicted that for silicene a band gap can be opened at the Dirac point with n-type doping by Cu, Ag, and Au adsorption, p-type doping by Ir adsorption, and neutral doping by Pt adsorption.[16] Peeters et al. studied the adsorption characteristics of alkali, alkaline-earth, and transition metal adatoms on silicene.[17] The adsorptions of Li, Na, and K result in the metalization of silicene, while the adsorptions of Be, Mg, and Ca turn silicene into a narrow gap semiconductor. Depending on the adatom type and atomic radius, the silicenes with adsorptions of transition metals can exhibit metal, half-metal, and semiconducting behavior respectively. Also, silicenes with adsorbed metal atoms, such as Li and K, are predicted to be promising host materials for hydrogen storage.[18,19] In addition, large adsorption energy and low diffusion energy barrier of Li atom on silicene suggest that silicene is also a suitable material for future anodes of lithium ion batteries (LIBs).[20] Note that the applications mentioned above need to avoid clustering the metal atoms when depositing metal atoms on silicene with high coverage. Therefore, the adsorption energy and diffusion energy barrier, which would affect the distribution of metal atoms on silicene, are important.
Up to now, silicene or silicene nanoribbons have been successfully fabricated on Ag,[21–27] Ir,[28] and ZrB2[29] substrates. Our previous study[30] showed that the O2 molecule dissociates on the silicene surface easily without overcoming any energy barrier, and the migration and desorption of O atoms are relatively difficult at room temperature due to the strong Si–O bonds. Therefore, the freestanding and pristine silicene is presumed to be unstably existent in the ambient condition. In contrast to the pristine silicene, full-hydrogenated silicene, so called silicane (SiH), may be realized more easily. Actually, silicene was initially synthesized by chemical exfoliation of calcium silicide (CaSi2),[31] where the silicon nanosheet is a monoatomic layer of silicon with a hexagonal crystal structure, and the surface of the monoatomic layer is capped with hydrogen. That is to say, the prepared silicene by using chemical exfoliation is in fact the silicane. Therefore, the study of the hydrogenated silicene should be essentially meaningful. Hussain et al.[32] and Yang et al.[33] have studied hydrogenated silicene functionalized with alkali and alkaline-earth metals for efficient hydrogen storage. In their studies, however, the metal atoms bind with silicane through substituting the hydrogen atoms with metal atoms. Strictly speaking, this is the interaction between metal atoms and defective silicane, not the adsorption of metal atoms on the pure silicane.
In order to explore the adsorption and diffusion behaviors of alkali and alkaline-earth metal atoms on the pure silicane, in this work, we calculate the adsorption energies and diffusion energy barriers of the alkali (Li, Na, K), alkaline-earth (Be, Mg, Ca) metal atoms on the silicane surface by using first-principles calculations. We also compare the results of the metal atoms on silicane with those on silicene. Our results show that the adsorption energies of all metal atoms considered in our calculations on the silicane are significantly smaller than those on silicene, and also smaller than the cohesive energy of metal. Furthermore, the diffusion energy barriers of the alkali metal atoms on silicane are comparable to those on silicene, whereas the barriers of the alkaline-earth are essentially smaller than those on silicene. Combining the results of the adsorption energies and the results of the diffusion energy barriers, it would be found that the clustering would occur when depositing metal atoms on hydrogenated silicene with relative high coverage.
Our total energy calculations are performed by using a plane-wave pseudopotential formulation[34] within the frame of density functional theory (DFT). The code is implemented in the Vienna ab initio Simulation Package (VASP).[35] The generalized gradient approximation (GGA) with the Perdew and Wang (PW91) exchange correlation functional[36] is employed. Interactions between electrons and ion cores are described by the projector augmented wave (PAW) potential.[37] A cutoff energy of 550 eV is employed for the plane wave expansion of the wave function. The Brillouin zone is sampled with 5×5×1 Monkhorst–Pack k-point mesh.[38] The convergence criteria for the total energy and ionic forces are set to be 10−5 eV and 0.01 eV/Å. Spin-polarized calculations are not employed in our calculations due to the non-magnetic results for the adsorbed silicene with Li, Na, K, Be, Mg, and Ca atom.[17]
In order to study the adsorption characteristics of alkali, alkaline-earth metal atoms on silicene or silicane, we employ a 4×4×1 unit cell in our calculations. There are 32 Si atoms in the unit cell of silicene, while 32 Si atoms and 32 H atoms in the unit cell of silicane. To eliminate the interaction emerging from periodic boundary conditions in the z direction, the closest distance between the layer and its periodic image is set to be larger than 11 Å. The metal atom migration pathway and energy barrier are optimized with the nudged elastic band (NEB) method[39] in our calculations.
The adsorption energy (Ead) of metal adatoms on a silicene or silicane sheet is determined by the following relation
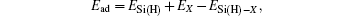
First, we examine the perfect structures of silicene and silicane. The optimized geometric structures are plotted in Fig.
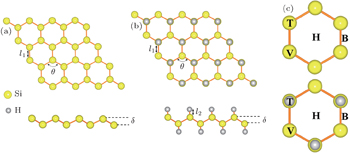 | Fig. 1. Optimized geometric structures of (a) silicene and (b) silicane; possible adsorption sites on the lattices of (c) silicene and (d) silicane. |
| Table 1. Structural parameters of silicene and silicane. a represents the lattice constant of an optimized unit cell, l1 and l2 are the Si–Si and Si–H bond lengths, respectively. δ and θ are the buckling distance and bond angle respectively, which are marked in Fig. |
Next, the adsorption behaviors of alkali metal atoms (Li, Na, K) and alkaline-earth metal atoms (Be, Mg, Ca) are investigated. Considering the symmetries of the silicene and silicane substrates, four different adsorption sites, namely, above the center of hexagonal silicon rings (hollow site H), at the top of the upper silicon atoms or hydrogen atoms (top site T), at the top of the lower silicon atoms (valley site V), and at the top of the Si–Si bond (bridge site B), are taken into account for the atom adsorption. The corresponding adsorption sites are marked in Fig.
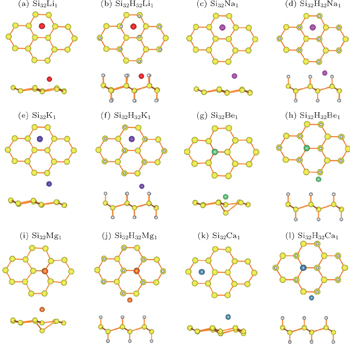 | Fig. 2. Side and top views of adsorption geometries for alkali and alkaline-earth metal atoms on silicene and silicane. |
In addition, the detailed total energies of silicene and silicane with adatoms at different initial adsorption sites are also provided in Table
| Table 2. Calculated results for alkali and alkaline-earth metal atoms adsorbed on silicene and silicane. Hollow (H), bridge (B), valley (V) and top (T) represent the adsorption sites. h is the adatom height, and δ is the buckling distance. Ead is the adsorption energy of adatom. . |
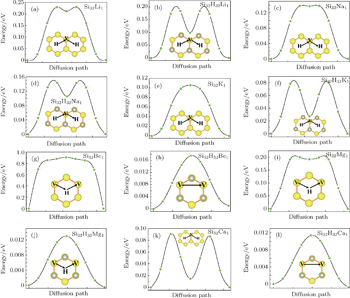 | Fig. 3. Diffusion energy barriers and diffusion paths for alkali and alkaline-earth atoms adsorbed on silicene and silicane. |
Next we discuss the adsorption energies of metal atoms on silicene. Our calculated adsorption energies of Li, Na, and K metal atoms, which are listed in Table
After the silicene is hydrogenated, thus forming silicane, the adsorption behaviors of metal atoms change a little. The adsorption sites of metal atoms are basically kept unchanged except for the case of Ca, in which the H site is changed into a V site. The buckling distance of silicane has little change after the atom adsorption. The adatom heights of Be, Mg, and Ca atoms on silicane are 3.711, 4.114, and 4.646 Å, respectively, each of which is significantly larger than that on silicene. However, the heights are close to those on silicene for the cases of Li, Na, and K. More importantly, the adsorption energies of all metal atoms on silicane are essentially lower than those on silicene. For a more convenient comparison, we arrange the adsorption energies of all metal atoms on silicane and silicene in one figure as seen Fig.
The diffusion behavior is another important issue for the adsorbed metal atoms. Therefore, we investigate the diffusion barriers of the metal atoms on different substrates, i.e., silicene and silicane, using the NEB method. The calculated energy profiles are plotted in Fig.
| Table 3. Optimized total energies of silicene and silicane with adatoms at different initial adsorption sites. . |
The diffusion barriers of the alkali metal atoms on silicane are extremely similar to those on silicene, about 0.20, 0.15, and 0.10 eV for Li, Na, and K respectively. In other words, hydrogenation does not change the potential surface of the silicene. Unlike the cases of alkali metal atoms, the diffusion barriers of the alkaline-earth metal atoms on silicane are significantly lower than those on silicene. Specifically, the diffusion barriers of Be, Mg, and Ca atoms on silicene are 0.91, 0.21, and 0.11 eV, whereas 0.02, 0.01, 0.01 V on silicane, respectively. Two factors result in the large difference. One is the local structure distortion after the adsorptions of alkaline-earth metal atoms on silicene, which impedes the movement of the metal atoms. This obvious distortion is not observed for the silicane substrate, thus reducing the barrier of the atom diffusion. The other factor is the weakened interaction between metal atoms and silicane, which is reflected by the adsorption energies. Weak interaction means little influence of the substrate on the movement of the adatom. Therefore, low diffusion barriers of alkaline-earth metal atoms on silicane could be understood clearly.
 | Fig. 4. (a) Adsorption energies and (b) diffusion energy barrier heights for alkali and alkaline-earth metal atoms adsorbed on silicene and silicane. |
Considering the relatively low adsorption energies and low diffusion barriers of alkali metal atoms and alkaline-earth metal atoms on silicane, it is likely for these atoms to form clusters. In order to prove this, we calculate the cohesive energies of the different bulk metals, which are listed in Table
| Table 4. Cohesive energies of the different bulk metals. . |
Actually, the results mentioned above correspond to the adsorption and diffusion of metal atoms on perfect silicane. If the silicane substrate is defective, the situations are significantly different. According to the study reported by Yang et al.,[33] the adsorption energies of Li, Na, and K atoms on silicane with hydrogen atoms replaced by adatoms are 2.507, 2.360, and 2.623 eV, respectively. These large adsorption energies ensure that Li, Na, and K atoms could be directly adsorbed on the silicane instead of forming metal clusters. Therefore, if we need to distribute the atoms uniformly on silicane, the hydrogen vacancies should be produced in advance. As for the adsorptions of Mg and Ca atom, only the adsorption energy of the Ca atom on silicane with a hydrogen atom replaced by an adatom is slightly larger than the corresponding cohesive energy of bulk metal. As a consequence, the alkaline-earth metal atoms are not suitable for the uniform distribution on silicane, regardless of perfect or defective silicane substrate.
We study the adsorption and diffusion behaviors of alkali (Li, Na, K) and alkaline-earth (Be, Mg, Ca) metal atoms on the perfect silicane by using first-principles calculations. At the same time, the obtained results are used to compare with that on silicene. Structurally, the effect of the metal atoms on the silicane is less than that on silicene. The adsorption energies of metal atoms on silicane are essentially lower than those on silicene, especially for the alkaline-earth metal atoms, indicating the weak interaction between the metal atoms and silicane substrate. According to our calculations, the diffusion energy barriers of the alkali metal atoms on silicane are similar to those on silicene. In contrast, the diffusion energy barriers of the alkaline-earth metal atoms on silicane are extremely low, all below 0.1 eV. Owing to the low adsorption energies, which are lower than the cohesive energies of the corresponding bulk metals, and the low diffusion energy barriers, the metal clusters are easy to form when depositing metal atoms on silicane. In order to avoid forming the metal cluster, we need to remove the hydrogen atoms from the silicane substrate to achieve the defective silicane.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 | |
| 39 | |
| 40 | |
| 41 | |
| 42 | |
| 43 |


