† These authors contribute to this work equally.
‡ Corresponding author. E-mail:
Project supported by the National Key Basic Research Program of China (Grant No. 2011CB302000), the National Natural Science Foundation of China (Grant Nos. 51232009 and 51202299), the Fundamental Research Funds for the Central Universities, China (Grant No. 11lgpy16), the Natural Science Foundation for Jiangsu Provincial Higher Education, Institutions of China (Grant No. 15KJB510005), and the Talent Fund of Jiangsu University, China (Grant No. 15JDG042).
Stable nitrogen doping is an important issue in p-type ZnO research for device applications. In this paper, beryllium and magnesium are systematically compared as a dopant in ZnO to reveal their nitrogen-stabilizing ability. Secondary ion mass spectrum shows that Be and Mg can both enhance the stability of nitrogen in ZnO while Be has a better performance. Zn 2p and O 1s electron binding energies change in both MgZnO and BeZnO thin films. Donor-acceptor luminescence is observed in the BeZnO samples. We conclude that Be is a better co-doping element than Mg for p-type ZnO:N.
ZnO has wide potential applications in ultra-violet optoelectronic devices mainly because of its band gap of 3.37 eV and exciton binding energy of 60 meV.[1–3] Fabrication of p-type ZnO, however, is still a main obstacle in achieving ZnO-based electroluminescence devices. Previous studies have shown that nitrogen as a doping element can produce shallow acceptor energy level and thus become a promising dopant for high hole concentration, high stability and reproducible p-type ZnO.[4,5] Direct doping of nitrogen into ZnO thin film is found to be very difficult in non-equilibrium growing experiments performed by most researchers. Co-doping is an effective way to solve this problem. Lithium, magnesium, gallium or other elements have been doped into ZnO together with nitrogen to acquire a more intense interaction between nitrogen and the lattice.[6–10] As isoelectronic cations in ZnO, beryllium and Mg have also attracted attention.[11] Wei et al. fabricated MgZnO:N by molecular beam epitaxy (MBE) and detected p-type conductivity after annealing.[12] Sanmyo et al. succeeded in fabricating Be–N co-doped ZnO with weak p-type conductivity by sputtering.[13] These results suggest the nitrogen-stabilizing effect of Mg and Be, which are not in agreement with calculations.[14,15] Comparison between the two neighboring group IIA dopants in ZnO helps to reveal their nitrogen-stabilizing ability, and provides guidance for further p-type doping. Differences in fabricating method and condition impede the systematic comparison between previous reports. Here we use molecular beam epitaxy, a clean growing method to avoid other disturbance, to fabricate MgZnO and BeZnO thin films under the same condition. By comparing our samples, we find that Mg and Be can both greatly enhance the stability of nitrogen in ZnO, while the nitrogen in BeZnO:N thin film stays stable even after high-temperature annealing. The changes in binding energies of Zn 2p and O 1s electrons imply an increase of oxygen content in the lattice. Luminescence from a donor–acceptor pair (DAP) is observed in BeZnO thin film. We deduce that the change in anion/cation ratio by doping affects the chemical potential of nitrogen and leads to the enhanced nitrogen stability. By comparing our results, we find that Be is better than Mg in nitrogen-stabilization. BeZnO would be a promising starting material for p-type doping.
The thin crystal films were grown on sapphire (0001) substrates by plasma-assisted MBE. Zn (6N), Be (4N) and Mg (6N) were supplied by solid source effusion cells. The O and N atoms were supplied from O2 and N2 gas through RF plasma generators. A designed buffer was employed to relieve the strain as much as possible and thus high crystal quality was achieved. The details of the buffer technique can be found in our previous work.[16] The doping content was controlled by the source temperature, i.e., higher source temperature corresponds to a higher doping level. In the nitrogen-doped samples, the flow rate was kept constant. The MgZnO:N thin films are n-type or highly resistant. The BeZnO:N thin films are highly resistant or weakly p-type, with a hole concentration of ∼ 1 × 1015/cm3 and a mobility of 2 cm2·V−1·S−1. The electrical properties of the films are stable at least for three months.
Secondary ion mass spectrum (SIMS) is used to investigate the relative nitrogen density in as-grown and annealed ZnO, BeZnO, and MgZnO films. Figure
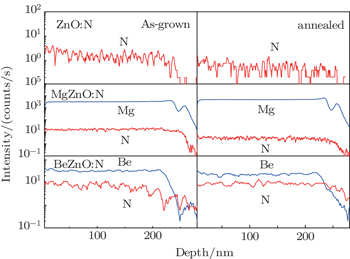 | Fig. 1. Secondary ion mass spectra for as-grown and annealed ZnO, BeZnO, and MgZnO doped with nitrogen. The content values of Mg and Be are ∼ 10% and ∼ 1% respectively. |
The high concentration of nitrogen in the as-grown MgZnO implies that nitrogen can be easily doped into the lattice during growth. But the nitrogen losses when the system process proceeds towards thermal equilibrium state. These results agree with those given by Gai et al., who predicted a higher formation energy of neutral defect NO in MgZnO than in ZnO, but succeeded to fabricate MgZnO:N by modifying chemical potentials during nonequilibrium growth.[6] On the other hand, nitrogen in BeZnO seems to be very stable even under thermal equilibrium state. However, this experimental fact is somewhat against the calculation which predicted that incorporating Be into ZnO:N can lower the ionization energy but disadvantage the stability of nitrogen.[15] To clarify the origin of this divergence, we reconsider the equation of formation energy used in calculation. The formation energy of neutral defect NO in ZnO is given by
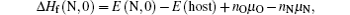
The chemical potential is strongly affected by stoichiometry of the lattice. Our previous work indicates that the anion/cation ratio in BeZnO alloy may change from 1 to over 1.1 by increasing Be doping. The positron annihilation spectrum also evidences that the oxygen vacancies are obviously suppressed by Be doping.[20] But no data have ever addressed that point for MgZnO. Here, on that point, we provide more detailed spectrum evidence for both BeZnO and MgZnO: photoluminescence (PL) and x-ray photoelectron spectrum (XPS) measurements are carried out. We compare samples with and without nitrogen doping to exclude the influence of nitrogen. It is found that no change is caused by nitrogen doping in the spectrum region we are interested in. This is because the nitrogen concentration is rather low compared with those of Be and Mg.
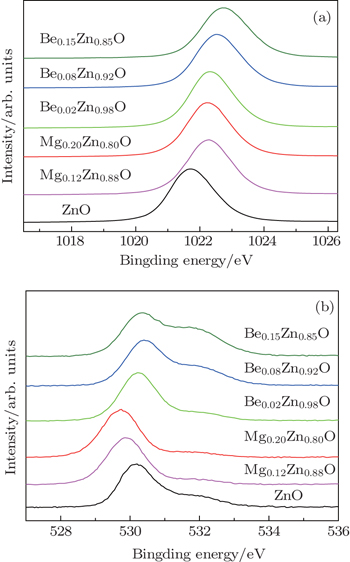
Figure
| Table 1. Oxygen content of ZnO films doped with different content values. . |
Modification of the O 1s binding energy by doping is also observed. As shown in Fig.
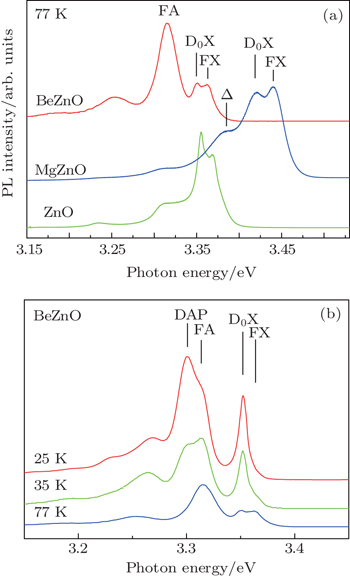
The PL spectra of ZnO, MgZnO, and BeZnO are shown in Fig.
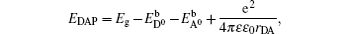
All the above evidence points to the same conclusion: the anion/cation ratios in BeZnO and MgZnO alloys increase and the anion/cation ratio is much more remarkable in BeZnO. This conclusion may be a way out for the problematic calculations mentioned above, because most of the calculated values of (E(N,0)–E(host)) are obtained on an assumed lattice with anion/cation ratio r = 1, which is no longer valid when the doping elements significantly affect the anion/cation ratio and break the balance of intrinsic defects. We suggest that it is more reasonable for the calculation work to take the variation of anion/cation ratio into account. The degree of the variation may take the experimental data as a reference. Whether this modification will do any favors to the model is still an open question waiting for its answer.
In this paper, we fabricate high-quality MgZnO:N and BeZnO:N alloy films by MBE under a similar growth condition. The nitrogen stability is confirmed by SIMS in both alloys. To study the nitrogen-stabilizing mechanism, we examine the BeZnO and MgZnO alloys in more detail by PL and XPS. In PL spectra, an additional donor–acceptor pair irradiative emission is observed in BeZnO while in MgZnO the emission signal is not so intense. This new emission peak implies the decrease of O vacancy and (or) the increase of Zn vacancy. On the other hand, XPS indicates that the content values of oxygen are increased in both alloys, which accords well with the implication from the PL spectrum. These phenomena are all related to the doping-induced modification of host element atomic ratio, which is not considered in calculations. Also from our experiments, the N stabilization and O vacancy suppression are both more prominent in BeZnO than in MgZnO. Thus Be is a better co-doping element than Mg for p-type ZnO:N.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 |