† Corresponding author. E-mail:
Project supported by the Youth Programs of Chongqing Three Gorges University, China (Grant No. 13QN18).
In this paper, based on the generalized heat transfer law, an air conditioning system is analyzed with the entropy generation minimization and the entransy theory. Taking the coefficient of performance (denoted as COP) and heat flow rate Qout which is released into the room as the optimization objectives, we discuss the applicabilities of the entropy generation minimization and entransy theory to the optimizations. Five numerical cases are presented. Combining the numerical results and theoretical analyses, we can conclude that the optimization applicabilities of the two theories are conditional. If Qout is the optimization objective, larger entransy increase rate always leads to larger Qout, while smaller entropy generation rate does not. If we take COP as the optimization objective, neither the entropy generation minimization nor the concept of entransy increase is always applicable. Furthermore, we find that the concept of entransy dissipation is not applicable for the discussed cases.
Air conditioning systems are widely used in many buildings. The analyses and optimizations of air conditioning systems have attracted more and more attention because they are very important for energy conservation and the reduction of cost.[1–10] In order to improve the system performance and increase the energy utilization, some optimization theories have been developed, such as the entropy generation minimization[11] and the entransy theory.[8]
The entropy generation minimization has been widely used in the analyses and optimizations of thermal systems.[12–17] In air conditioning systems, this theory has also been applied.[18,19] However, some researchers further found that the entropy generation minimization does not always lead to the optimal performances for different optimization objectives. For example, when the refrigeration capacity is not fixed, Klein and Reindl[20] found that minimizing the entropy generation rate does not always result in the same design as maximizing the system performance, and when larger heat flow into the high temperature heat source is the objective, the entropy generation minimization is not always applicable, either.[8]
The entransy theory was proposed by Guo et al.[21] for heat transfer and it has been widely applied to analyzing heat transfer processes.[21–32] This theory has also been extended to the analyses of thermodynamic processes.[33–36] Cheng and Liang[33] defined a new concept of entransy loss rate. For the discussed systems, it was shown that the entransy loss rate maximum results in the output power maximum.
For the heat pump system, Chen et al.[18] applied the entropy concept to its optimization. But Klein and Reindl[20] found that the entropy generation rate minimum does not always result in the best system performance. So the discussion of the applicability of the entropy generation minimization to the heat pump systems is still necessary. Recently, Cheng and Liang[8,10,19] used the entransy theory to analyze air conditioning systems. They found that the concept of entransy increase is applicable for the analyses of the heat flow into the high temperature heat source. However, there are not many papers on the analyses of air conditioning systems with the entransy theory. Further investigations are still necessary.
In this paper, based on the generalized heat transfer law, an air conditioning system is analyzed with the entransy theory and the entropy generation minimization. The coefficient of performance (denoted as COP) and the heat flow rate Qout which is released into the high temperature heat source are taken as the optimization objectives, and the applicabilities of the entropy generation minimization and the entransy theory to the optimizations are also discussed.
In the analogy between the electrical and heat conductions, Guo et al.[21] noted that the thermal system lacks the parameter corresponding to the electrical potential energy. With this consideration, they proposed the concept of entransy, which can be expressed as[36,37]
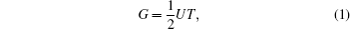
Furthermore, Cheng and Liang[33] and Cheng et al.[34] proposed the concept of work entransy, with which the entransy balance equation for thermodynamic cycles can be set up. Then, the concept of entransy loss was developed.[33] For a thermodynamic system, it was shown that entransy loss is the difference between the entransy flows that get into and out of the system, and the consumed entransy.[33,37] When there is no work done in the thermodynamic system, the thermodynamic system becomes a heat transfer system, and the entransy loss equals the entransy dissipation.[37] Cheng and Liang[33] found that larger entransy loss leads to larger output work when some conditions are satisfied. In some application cases, the concept of entransy loss was found to be effective for the analyses and optimizations of some thermodynamic systems.[33–37,45–48]
However, some researchers[49,50] thought that the entransy theory was a copy of the entropy generation minimization, or gave the same optimization results as those of the entropy generation minimization. In heat transfer and thermodynamic cycles, many comparisons showed that the two theories are different.[22–24,33,34,37] We also did some work on the analyses of an endoreversible Carnot heat engine with the entransy theory and the entropy generation minimization.[51] The results showed that the two theories are not the same, and the major problems in Ref. [51] were pointed out. In the present work, this issue will also be discussed in the analyses of an air conditioning system.
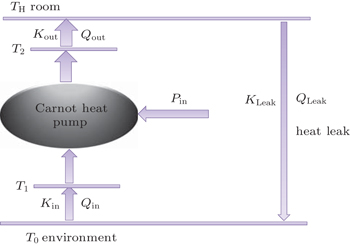
The discussed air conditioning system is shown in Fig.
In this system, the heat flow rate Qin, which is from the environment at temperature T0, flows into the Carnot heat pump, which works between temperatures T1 and T2. During the cycle, the input power is Pin, and the heat flow rate Qout is released to the room at temperature TH. The heat leak rate QLeak between the environment and the room is considered.
We assume that the heat transfer processes satisfy the generalized heat transfer law,[52–55] there are
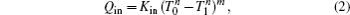
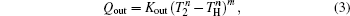
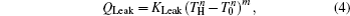
Considering the energy conservation, there is
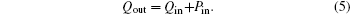
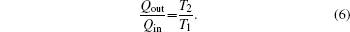
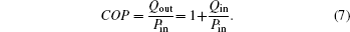
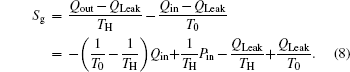
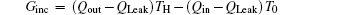
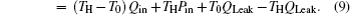
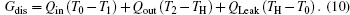
Taking COP and Qout as the optimization objectives, we analyze the relationships of COP, Qout, Sg, Ginc, and Gdis below. Five cases will be calculated numerically.
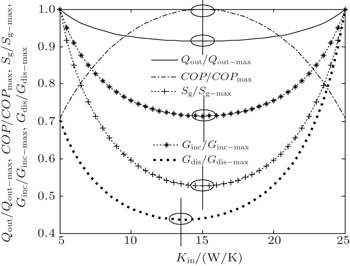
In the first case, we assume that TH = 300 K, T0 = 275 K, and Qin = 200 W. When m = n = 1, the heat transfer law is the Newton heat transfer law. With this law, we assume KLeak = 0.8 W/K and Kin + Kout = 30 W/K. As Kleak, Kin, and Kout are the products of the heat transfer areas and the corresponding heat transfer coefficients, their values depend on the size of the heat pump and the corresponding heat transfer performance. So, the values may be different for different heat pumps. In this paper, the data are set to be close to those in Ref. [8]. With Eqs. (
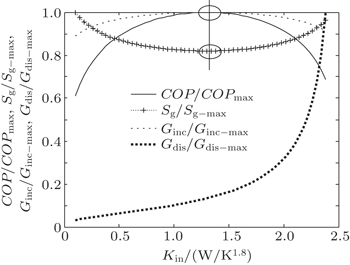
It can be seen that COP reaches its maximum value for Kin = 15.0 W/K, while Qout, Sg, and Ginc reach their minimum values. However, Gdis reaches neither its minimum value nor its maximum value at the same point. Furthermore, we can calculate two more heat transfer laws. When we assume that m = 1.2, n = 1.5, KLeak = 1 × 10−2 W/K1.8, and Kin + Kout = 2 W/K1.8, the variations of COP, Qout, Sg, Ginc, and Gdis with Kin can be shown in Fig.
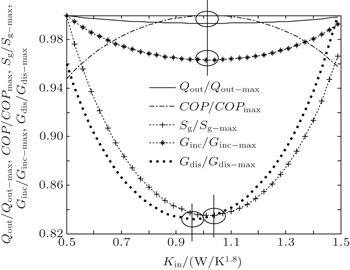 | Fig. 3. Variations of COP, Qout, Sg, Ginc, and Gdis with Kin when Qin is fixed, m = 1.2 and n = 1.5. |
In this case, Qin, TH, T0, and QLeak are all given. We can find that the variation tendencies of Qout, Sg, and Ginc are the same as that of Pin in Eqs. (
As above, we show that both the entropy generation minimization and the entransy increase minimization lead to the maximum COP in this case. On the other hand, if larger Qout is our objective, larger entransy increase rate leads to larger Qout, while smaller entropy generation rate does not. This conclusion is consistent with that obtained by Cheng and Liang[8] when they analyzed a heat pump system with the concepts of entropy generation and entransy increase. Furthermore, our results show that the concept of entransy dissipation is not applicable for the optimizations, and this conclusion is consistent with that obtained by Cheng et al.[37,55] when they reviewed the entransy theory.
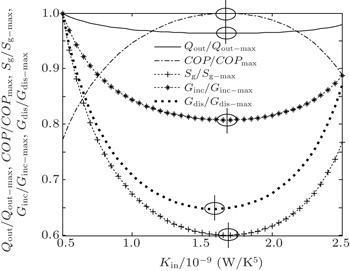
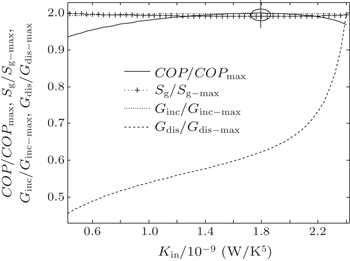
In the second case, we assume that TH = 300 K, T0 = 275 K, and Pin = 300 W. With Eq. (
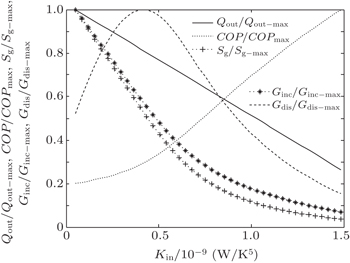
When m = 1.2 and n = 1.5, we assume that KLeak = 1 × 10−2 W/K1.8 and Kin + Kout = 2.5 W/K1.8. When m = 1 and n = 5, we assume that KLeak = 1 × 10−10 W/K5 and Kin + Kout = 2.5 × 10−9 W/K5. For these two heat transfer laws, the variations of COP, Sg, Ginc, and Gdis with Kin are shown in Figs.
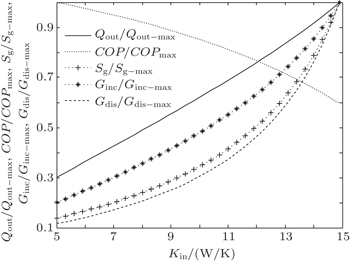
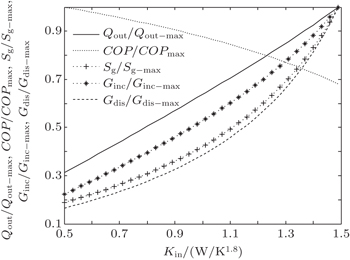
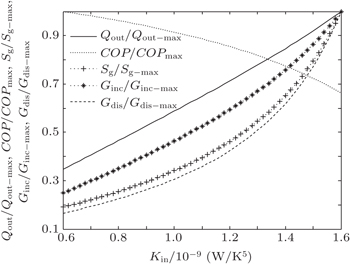
In the third case, we assume that TH = 300 K, T0 = 275 K, and T1 = 265 K. The three heat transfer laws are also discussed here. We assume that KLeak = 0.5 W/K and Kin + Kout = 20 W/K for m = n = 1, KLeak = 5 × 10−2 W/K1.8 and Kin + Kout = 2 W/K1.8 for m = 1.2 and n = 1.5, and KLeak = 5 × 10−11 W/K5 and Kin + Kout = 2 × 10−9 W/K5 for m = 1 and n = 5, respectively. For the three heat transfer laws, the variations of COP, Qout, Sg, Ginc, and Gdis with Kin can be shown in Figs.
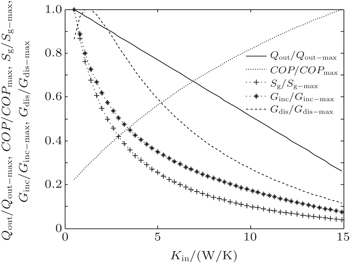
In the fourth case, we assume that TH = 300 K, T0 = 275 K and T2 = 325 K. For the three heat transfer laws, we assume that KLeak = 1 W/K and Kin + Kout = 20 W/K when m = n = 1, KLeak = 0.1 W/K1.8 and Kin + Kout = 2 W/K1.8 when m = 1.2 and n = 1.5, and KLeak = 1 × 10−10 W/K5 and Kin + Kout = 2 × 10−9 W/K5 when m = 1 and n = 5, respectively. The variations of COP, Qout, Sg, Ginc, and Gdis with Kin are also shown in Figs.
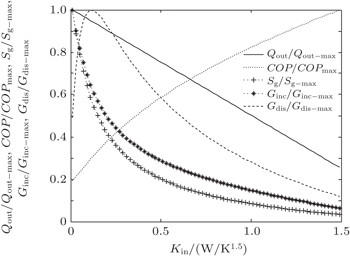 | Fig. 12. Variations of COP, Qout, Sg, Ginc, and Gdis with Kin when T2 is fixed, m = 1.2 and n = 1.5. |
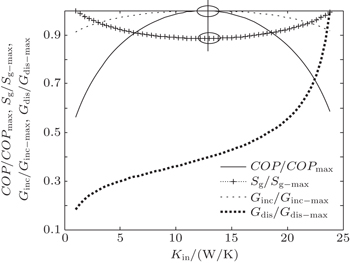
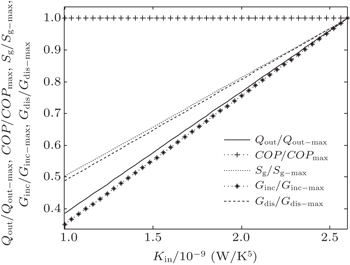
In the fifth case, we assume that TH = 300 K, T0 = 265 K, T1 = 263 K, and T2 = 320 K. According to Eqs. (
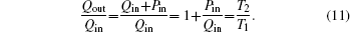
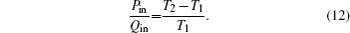
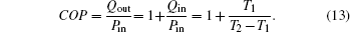
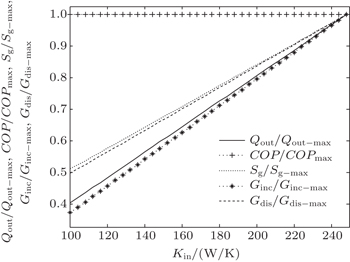 | Fig. 14. Variations of COP, Qout, Sg, Ginc, and Gdis with Kin when T1 and T2 are fixed and m = n = 1. |
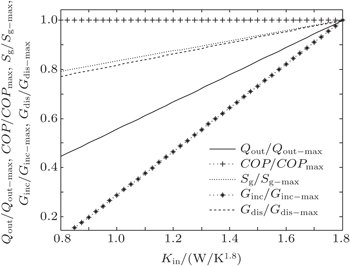 | Fig. 15. Variations of COP, Qout, Sg, Ginc, and Gdis with Kin when T1 and T2 are fixed, m = 1.2 and n = 1.5. |
The results show that Qout, Sg, Ginc, and Gdis all increase monotonically with increasing Kin. Therefore, larger entransy dissipation rate, larger entropy generation rate, and larger entransy increase rate all lead to larger Qout. For the analyses of COP, none of the concepts of entransy dissipation, entransy increase and entropy generation are applicable.
In this paper, the applicabilities of the entropy generation minimization and the entransy theory to the optimization analysis of an air conditioning system are discussed. Three different heat transfer laws and five cases are analyzed numerically.
The applicabilities of the entransy theory and the entropy generation minimization to the discussed air conditioning system are conditional. If larger Qout is the objective, larger Ginc always leads to larger Qout, while smaller entropy generation does not. If COP is taken as the optimization objective, neither the entropy generation minimization, nor the entransy increase minimization (or maximization) nor the entransy dissipation minimization (or maximization) always leads to the maximum COP.
For the five discussed cases, the concept of entransy dissipation is not applicable for the optimizations of COP nor Qout.