† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant No. 51172089), the Program for New Century Excellent Talents in University, the Natural Science Foundation of Guizhou Provincial Education Department (Grant No. KY[2013]183), and the Collaborative Fund of Science and Technology Office of Guizhou Province, China (Grant No. LH[2015]7232).
The effect of the catalyst height on the morphology of diamond crystal is investigated by means of temperature gradient growth (TGG) under high pressure and high temperature (HPHT) conditions with using a Ni-based catalyst in this article. The experimental results show that the morphology of diamond changes from an octahedral shape to a cub-octahedral shape as the catalyst height rises. Moreover, the finite element method (FEM) is used to simulate the temperature field of the melted catalyst/solvent. The results show that the temperature at the location of the seed diamond continues to decrease with the increase of catalyst height, which is conducive to changing the morphology of diamond. This work provides a new way to change the diamond crystal morphology.
Diamond, as the hardest substance in the natural world, is of great importance in science and technology with many applications, such as a fine grinding material, high hardness cutting tools and parts of precision instruments. Since diamond was first synthesized in a metal catalyst/solvent and graphite system by using the high pressure and high temperature (HPHT) method in 1954,[1] numerous studies of its growth mechanism and outstanding properties have been conducted. According to the abundant research results of pioneers, the catalyst and solvent have been recognized to play a critical role in synthesizing diamond when using the HPHT method.[2–4] Different kinds of catalysts have been found and studied to explore the influence on diamond synthesis with using the HPHT method. The non-metallic catalysts generally require HPHT conditions and thus the obtained diamond is of a lower quality. However, metallic solvent catalysts could obtain sufficiently large crystals of synthetic diamond with high purity and high quality in a relatively mild condition.[5–16] Nevertheless, little attention has been paid to researching the effect of the height of the catalyst itself on the growth process of diamond. In consideration of the important role of the catalyst, we here focus on the catalyst based on Ni-based alloy to explore the effect of catalyst height on the growth process of diamond crystal.
It is well known that the temperature gradient growth (TGG) under HPHT conditions is an effective method to synthesize large single crystal diamond.[17–22] We also knew that the temperature gradient is a driving force of diamond growth. However, it is very difficult to ascertain the dependence of the temperature characteristic in the catalyst on the catalyst height. Therefore, the understanding of the temperature fields of different heights of catalyst is the key to investigating the effect on diamond perfection and morphology. The finite element method (FEM) is a powerful calculation tool[23,24] for understanding the temperature characteristic in high-pressure synthesis cells.
In this study, large diamond crystals are synthesized by using different heights of catalyst by TGG under HPHT conditions. Our work is to clarify the effect of catalyst height on the crystal morphology of diamond. The FEM is used to simulate the melted catalyst and solvent temperature field of the growth cell, and thus explaining the growth mechanism of diamond crystals. We believe that our work would be helpful and meaningful for synthesizing different shapes of single crystal diamonds, which are used for industrial production and scientific research.
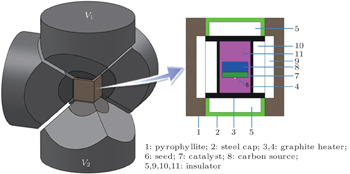
Experiments on diamond crystallization were carried out in a china-type large cubic high-pressure apparatus (CHPA) with a sample assembly of 38 mm×38 mm×38 mm at a pressure of 5.7 GPa. The sample assembly for synthesizing diamond by the temperature gradient method (TGM) is shown in Fig.
After the HPHT experiments, the products were dissolved in a hot mixture of HNO3 and H2SO4 to remove the impurities remaining on the surfaces of the crystals. Morphologies of the synthesized samples were characterized by optical microscopy.
The distribution of temperature field in the melted catalyst and solvent of the growth assembly was simulated by FEM. We chose solid 69 for thermal-electrical analysis. The finite element model, boundary conditions, and material parameters were cited from other previous reports.[25,26]
Figure
Figure
 | Fig. 2. Synthesized diamond crystals in different heights of Ni-based catalyst: (a) 2.0 mm, (b) 2.4 mm, (c) 2.8 mm. |
It is well known that the morphology of diamond changes from cubic crystal mainly with {100} crystal faces, cub-octahedral crystal mainly with {100} and {111} crystal faces to octahedral crystals mainly with {111} crystal faces with the increase of synthetic temperature in the diamond process by TGG under HPHT. In the pressure–temperature (P–T) phase diagram of carbon, the region for diamond growth is a V-shape region bounded by a growth-graphite equilibrium line and solvent and carbon eutectic melting line in the metal solvent-carbon system. As shown in Fig.
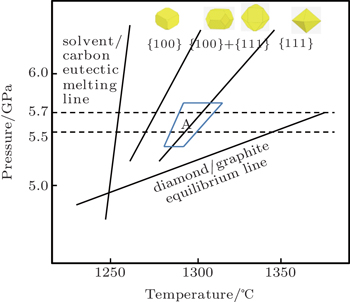 | Fig. 3. P–T phase diagrams of diamond and graphite crystallization in the Ni–Mn–Co–C system (Region A: P and T for our obtained diamond crystals by the HPHT method). |
Region A in Fig.
As is well known, based on the TGG under HPHT conditions of the diamond growth process, graphite powder is placed in the high temperature region, and a diamond seed is embedded in the low temperature region. When the cell is heated electrically by the graphite heater, the graphite powder is converted into diamond in a few minutes after a given temperature has been reached under the high pressure. We also know that diamond has a high thermal conductivity of about 2000 W/m·K, which is much higher than the Ni-based catalyst. When the pressure, voltage, and dimension of the graphite heater are fixed, the heat quantities generated by the graphite heater are the same in experiments. Owing to the high thermal conductivity of diamond, the temperatures in carbon source are almost the same. Furthermore, when using a thick catalyst, the temperature difference between the carbon source and the surface of the seed diamond is larger than when using a thin catalyst. Thus, as the temperature of the carbon source in the high temperature region is almost unchanged, it leads to the lower temperature of seed diamond in the low temperature region. That is to say, the synthesis temperature decreases with the increase of catalyst height.
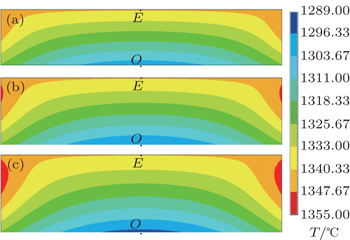
Based on the above experimental results, the morphologies of diamond crystals change as the catalyst height changes. In order to analyze the effect of height of catalyst on the morphology of diamond, FEM is used to simulate the temperature field of the catalyst in the process of diamond growth. As shown in Fig.
Figure
The diamond morphology is obviously affected by the melted catalyst and solvent heights in the large diamond growth process. The experimental results show that the morphology of the diamond changes from an octahedral shape to a cub-octahedral shape as catalyst height rises. Numerical simulation results show that the temperature in the seed diamond continues to decrease with the increase of catalyst height. The numerical simulation results can be used to explain some details of the diamond growth process. Our results not only provide a promising route to the adjustment of diamond synthesis assembly, but also offer an effective solution for commercial production of different shapes of large diamond crystals.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 |