† Corresponding author. E-mail:
‡ Corresponding author. E-mail:
Y2O3:Eu3+ phosphors co-doped with different metal cations (Li+, Na+, K+, Mg2+, Ca2+) are prepared by the gel-combustion method with Y2O3, Eu2O3, and R(NO3)x (R = Li, Na, K, Mg, Ca) serving as raw materials and glycine as fuel, calcined at 1000 °C for 2 h. The synthesized Y2O3:Eu3+ phosphors doped with different metal cations and doping ratios are characterized by x-ray diffractometry (XRD), fluorescence and phosphorescent spectrophotometer. The co-doping metal cations are advantageous to the development of Y2O3:Eu3+ lattice. All the samples can emit red light peaked at 611 nm under 254-nm excited. The luminescence intensities of co-doping samples are increased because the cations increase the electron transition probability of Eu3+ from 5D0 level to 7F level. The fluorescence lifetime of Eu3+ (5D0 → 7F2) is increased by doping metal cations.
Yttria has been widely used as the matrix material due to its fascinating properties, such as high chemical stability, high fusing point, good thermal stability and good optical performance. Yttria doped with Eu3+ ions is a kind of red fluorescent material with high luminous efficiency. It has good performance in the fields of cathode ray tube, field emission display, plasma display, etc.[1–3]
Alkali metal has been used as an additive material in order to improve the performances of materials.[4–8] The doped ions could enhance the transition probability of the rare earth ions. The luminescence intensities of SrAl4O7:Mn4+, SrZn2(PO4)2:Tb3+, Sr3B2O6:Ce3+, Eu2+, BaZn2(PO4)2:Sm3+, and AMgPO4:Eu3+ increased when the alkali metal ions were added. The color purity also increased.[9–13] The alkali metal ions co-doped Y2O3:Eu3+ phosphors with the quenching concentration achieving up to 12% mol were prepared and studied in Ref. [14]. As Na+ and K+ ions were added, the photoluminescence intensity of the Y2O3:Eu3+ TFP was improved by 3 to 4 times. The lifetime of Na+ and K+ co-doped Y2O3:Eu3+ thin film could be adjusted by the molar ratios of Na+ and K+ ions.[15] References [16] and [17] reported that the luminescence intensities of samples doped with zinc increased. The phosphors were more suitable for the usage of field emission display.
The alkaline earth ions have the similar properties to alkali metal ions. The valence state of the alkali metal ion is +1, and that of the alkaline earth metal ion is +2. The difference of valence state between the metal ions and rare earth elements gives rise to a distortion of the rare earth oxides crystal field, affects the development of a lattice, and improves the optical performances of the materials. In the present study, Y2O3:Eu3+ phosphors co-doped with different alkali and alkaline earth metal ions are prepared by using the gel-combustion method, with glycine used as the fuel. The crystalline phase and optical properties of samples doped with different metal cations are analyzed. The energy level transitions of Eu3+ doped with different metal cations are also discussed in the paper. The luminescence properties and fluorescence lifetimes of samples with different doping concentrations of metal cations are studied.
All raw materials used for synthesizing phosphors were of analytical reagent grade. A stoichiometric ratio of solid oxides of yttrium and europium was dissolved in aqueous nitric acid for 0.5 mol/L. The doping concentration of Eu3+ was 5% mol. The metal nitrates (LiNO3, NaNO3, KNO3, Mg(NO3)2, Ca(NO3)2) and glycine as the fuel were dissolved in the rare earth nitric solution under continuous stirring for 1 h and the molarity ratio of cations and glycine was 1:3.33. The transparent gel was obtained after the solution had been heated in the water bath at 80 °C for 8 h and dried at 100 °C for 24 h. When the dry gel was heated at 400 °C, the combustion reaction took place and the puffy precursor powders were obtained. (NH4)2SO4 as the dispersant and the precursor were stirred with deionized water for 1h and dehydrated at 80 °C for 24 h. Y2O3:Eu3+ powders co-doped with metal cations were obtained by calcining the dispersed precursor at 1000 °C for 2 h.
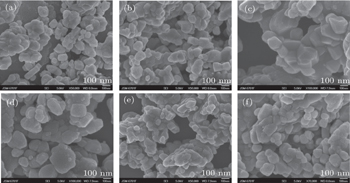
The x-ray diffraction (XRD) patterns were performed using a Rigaku D/max 2500 PC diffractometer in steps of 0.02° with a duration of 2 s per step in the 2θ range from 10° to 80°, and Cu Kα1 (λ = 0.15406 nm) was used as a radiation source, with an accelerating voltage of 20 kV and a working current of 10 mA. The JSM-6701F field emission scanning electron microscopy (SEM) was used to inspect the microstructures and morphologies of samples. The luminescence properties of samples were characterized by a Shimadzu RF-5301PC fluorescence spectrophotometer under the excitation of 254-nm ultraviolet light. The luminescence decay curves were obtained by phosphorescent spectrophotometer.
Figure
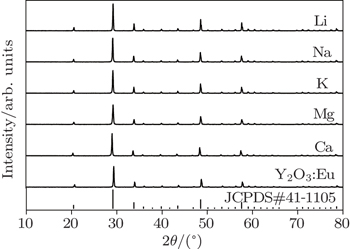 | Fig. 1. XRD patterns of samples doping with different cations (with Eu3+ and metal cations concentrations of 5% and 1%). |
According to XRD patterns of samples doped with different metal cations, the lattice parameters of samples can be calculated. Only one lattice constant needs to be calculated due to the fact that the Y2O3 is a cubic crystal and the lattice constants a, b, and c are identical. The lattice constants of Y2O3 and samples doped with Li+, Na+, K+, Mg2+, and Ca2+ ions in the plane (222) are shown in Table
| Table 1. Lattice constants and grain sizes of samples doped with different cations. . |
Figure
Figure
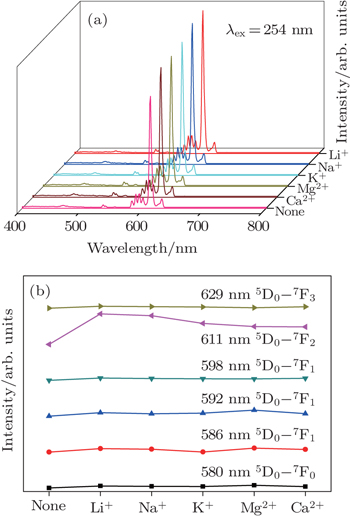
The 580-nm emission peak of Eu3+ is from the 5D0 → 7F0 transition, the 586-nm, 592-nm, and 598-nm peaks are from the 5D0 → 7F1 transition, and the 629 peak is from the 5D0 → 7F3 transition. The strongest emission peak at 611 nm is from the 5D0 → 7F2 transition. The 254-nm excited light of Y2O3:Eu3+ phosphor is located in the charge transfer band. The emitting intensities of samples are sensitive to the change of electric charge. As shown in the figure, the intensities of co-doped samples at the peak of 611 nm increase significantly and the differences in emission intensity variation among other peaks are small. The 5D0 → 7F2 transition is called the hypersensitive transition which is sensitive to the change of the surrounding. The doping ions, the doping metal cations, make a tiny distortion of the Y2O3 crystal field so that the intensity of 611-nm increases obviously.
Figure
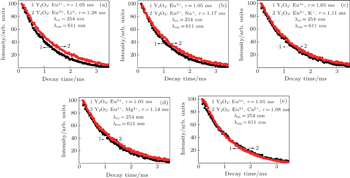
The decay curves for the luminescence of Eu3+ in samples doped with different cations are shown in Fig.
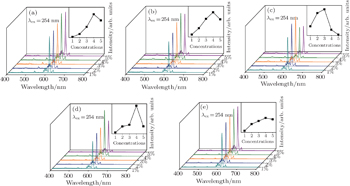
In this paper, Y2O3:Eu3+ phosphors co-doped with different metal cations are prepared by the gel-combustion method with Y2O3, Eu2O3, and R(NO3)x (R = Li, Na, K, Mg, Ca) as raw materials and glycine as fuel, calcined at 1000 °C for 2 h. The doping metal cations make a tiny distortion of the Y2O3 crystal field and do not change the cubic phase at all. The co-doped metal cations play a role in being the fluxing agent and are advantageous to the Y2O3:Eu3+ lattice. The diffraction peak intensity of a sample co-doped with Li+ is strongest. All the samples can emit red light peaked at 611 nm under the excitation of 254-nm ultraviolet light. The co-doped metal cations increase the transition probability of electrons from 5D0 to 7F2 energy level more than to other levels. The intensities of doped samples at the peak of 611 nm increase significantly and the differences in emission intensity variation among other peaks are small. The luminescent intensities of K+ co-doped sample with the content of 3% mol and the other co-doped samples with the content of 4% mol are strongest. The fluorescence lifetime of Eu3+ (5D0 → 7F2) increases by doping metal cations. With the luminescent intensities increasing, the decay times of samples also increase.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 |