† Corresponding author. E-mail:
Project supported by the Ibnu Sina Institute for Scientific and Industrial Research, Physics Department of Universiti Teknologi Malaysia and the Ministry of Education Malaysia (Grant Nos. Q.J130000.2526.04H65).
In this study, nanocrystalline Co–Ni–Mg ferrite powders with composition Co0.5Ni0.5−xMgxFe2O4 are successfully synthesized by the co-precipitation method. A systematic investigation on the structural, morphological and magnetic properties of un-doped and Mg-doped Co–Ni ferrite nanoparticles is carried out. The prepared samples are characterized using x-ray diffraction (XRD) analysis, Fourier transform infrared spectroscopy (FTIR), field emission scanning electron microscopy (FESEM), and vibrating sample magnetometry (VSM). The XRD analyses of the synthesized samples confirm the formation of single-phase cubic spinel structures with crystallite sizes in a range of ∼ 32 nm to ∼ 36 nm. The lattice constant increases with increasing Mg content. FESEM images show that the synthesized samples are homogeneous with a uniformly distributed grain. The results of IR spectroscopy analysis indicate the formation of functional groups of spinel ferrite in the co-precipitation process. By increasing Mg2+ substitution, room temperature magnetic measurement shows that maximum magnetization and coercivity increase from ∼ 57.35 emu/g to ∼ 61.49 emu/g and ∼ 603.26 Oe to ∼ 684.11 Oe (1 Oe = 79.5775 A·m−1), respectively. The higher values of magnetization Ms and Mr suggest that the optimum composition is Co0.5Ni0.4Mg0.1Fe2O4 that can be applied to high-density recording media and microwave devices.
The wide interest in ferrite nanoparticles is due to their unique and unusual chemical and physical properties with their high surface activities. A key factor for improving the properties of these particles due to their miniature size lies in enhancing the fraction of their surface atoms. It is useful in many applications such as recording media, microwave devices, pigments, high frequency cores, high-density magnetic information storage, absorbents, and sensors.[1–3]
Nanomaterials are mostly considered as materials in which the sizes of the particles or crystalline are below 100 nm in at least one or more dimensions. On a nano scale, a huge fraction of the atoms exist at (or close to) the surfaces of particles, which causes the unique characteristics of materials. Nanomaterials each have a large surface-to-volume ratio thus often exhibit amazing properties that differ from bulk ones. This type of structure belongs to a large class of compound that has a spinel type of structure. The structural and magnetic properties of nanocrystalline ferrites are affected by a variety of factors including processing conditions, sintering temperature, precursor atmosphere, chemical composition, cation distribution in the tetrahedral (A) and octahedral [B] sites, grain size, voids, surface layers, and doping.[4–7]
Spinel ferrites are made up of a regular combination of oxygen with the general formula of (A)[B]2O4. A crystal structure of spinel ferrite with the Fd-3m space group has 56 atoms. It contains 32 oxygen anions; 8 of 64 tetrahedral (A-site) and 16 of 32 octahedral interstitial sites (B-site) are filled by metal cations. The A-site cations are present in the interstices between 2 interpenetrating FCC lattices, while the B site cations reside in the interstices among 4 interpenetrating FCC lattices.[8,9] This special structure allows different metallic ions to be introduced to change its properties such as magnetic, electronic and other properties considerably.[10,11] A multitude of studies have focused on NiFe2O4 doped with other metal ions that provide improved magnetic properties for applications in the industry and medicine.[12,13]
Recently, the effect of Mg-doping on NiCoZnFe2O4 was studied and the coercive field was reported to decrease as a result of Mg doping. The maximum saturation magnetization (Ms) is present at x = 0.05.[14] Wang et al. studied the structural and magnetic properties of Mg-doped Co spinel ferrite and they found that Ms of Co1−xMgxFe2O4/SiO2 ferrite decreases with increasing Mg content.[15] It was reported that with the increase of MgO doping in Ni1−xAxFe2O4 nanoparticles, both Ms and Hc increase to 51.53 (emu/g) and 98.64 (Oe), respectively.[16]
There are several studies focusing on the effects of other co-substituted ferrites. The influences of magnetic ion substitution such as Ni2+,[17] Mn2+,[18] Gd3+,[19] and Cr3+ [20] on structural, magnetic, electric, and dielectric properties of CoFe2O4 have been investigated. Nevertheless, several researchers have reported on non-magnetic ions such as Al3+,[21] Y3+,[22] Zn2+,[23] Cu2+ [24] or Cd2+ [25] substituting CoFe2O4 and very few detailed studies of the Mg2+. Magnesium ions with non-magnetic nature are known for achieving control over magnetic parameters in developing magnetic recording technology and they have a preference for strong B sites. Akther Hossain et al. observed that when the non-magnetic divalent cations such as Zn, Mg, are substituted for magnetic cations such as Ni, Co, Mn, the maximum magnetization (Ms) as well as the real part of the initial permeability (m = i) increases up to 50%.[26] Ni1−xMgxFe2O4 was synthesized by John Berchmans et al. using citrate gel process. They suggested that Mg2+ ions cause appreciable changes in the structural and electrical properties of the ferrites.[27]
Motivated by these studies, we investigate the effects of magnesium concentration on the structural, morphological and magnetic properties of Mg substituted Co–Ni ferrites synthesized using the co-precipitation method sintered at 900 °C for 10 h. The improvement mechanism of the structural and magnetic properties is analyzed in detail.
In our study, the chemical reagents for this experiment are nickel nitrate [Ni(NO3)2.6H2O], magnesium nitrate [Mg(NO3)2.6H2O], cobalt acetate [Co(COO)2.4H2O], and iron nitrate [Fe(NO3)3.9H2O]. For sample preparation, initially the raw materials are prepared according to the prescribed composition ratios in the compound to be fabricated, and then are dissolved in de-ionized water. The aim of this step is to prepare the solution with the required concentration. A beaker and magnetic stirrer are used to mix these solutions constantly and then they are heated up to 80 °C. The NaOH solution (2.0 M) is added dropwise into the reaction mixture for precipitation and the pH is kept in a range of approximately 13. De-ionized water is used to wash the precipitates four times to remove the impurities, and then they are immediately dried at a temperature of 150 °C in an electric oven for water content removal purposes. These precipitates are then annealed in air at 900 °C for 10 h with a heating rate of 5 °C/min. Nanocrystalline Co0.5Ni0.5−xMgxFe2O4 powders with different crystallite sizes are prepared.
For confirmation of the spinel phase structure and the sample purity, the samples are characterized using an x-ray diffractometer (XRDM, D8 Advanced) with Cu Kα radiations λ = 1.5406 Å at 40 kV and 40 mA. The speed of scanning is set at ∼ 2 °C/min with a resolution of 0.011 and 2θ scanning range from 20° to 70°. Field emission scan electron microscopy (FESEM) images are recorded using FESEM JEOL, JSM-6701F, operated at 5 kV. The elemental analysis is made by energy dispersive x-ray spectroscopy (EDX) attached with FESEM. Fourier transform infrared (FTIR) spectra are recorded using Perkin-Elmer 5DX FTIR after mixing 1 mg of the ferrite sample with 100 mg of potassium bromide (KBr). They are thoroughly mixed in mortar for 5 min until a fine mixture is obtained. Pellets of 10-mm diameter are then prepared from these samples. Hysteresis loops are measured using a vibrating sample magnetometer (VSM, LakeShore-7404) at room temperature with a maximum applied field of 12 kOe.
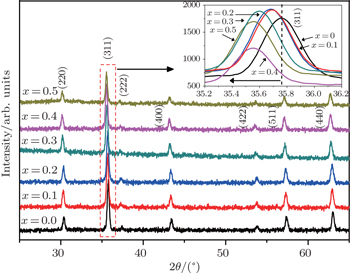
The room temperature x-ray diffraction (XRD) patterns of samples are demonstrated in Fig.

| Table 1. Characteristic parameters for each composition at room temperature. . |
Figure
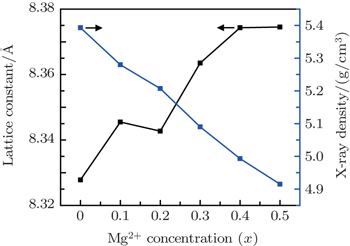
Vegard’s law is used to explain the relationship between Mg2+ substitution and lattice parameter.[33] The lattice constant (a), cell volume (V), and the x-ray density are calculated from the XRD spectra through using the following relations:
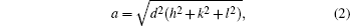

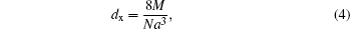
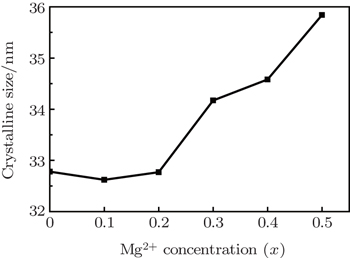
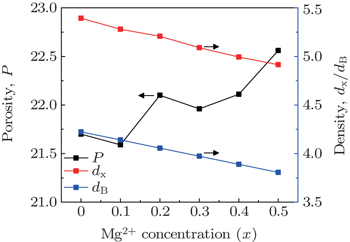
It is observed from Fig.
The diameter of particle and the bulk density are used to estimate the specific surface area (S) from the following relation[34]
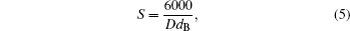
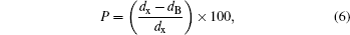
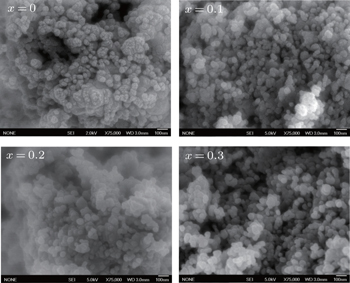
The field emission scan electron microscopy images of the co-precipitated Co0.5Ni0.5−xMgxFe2O4 ferrite samples sintered at 900 °C are shown in Fig.
The EDX analyses (Figs.
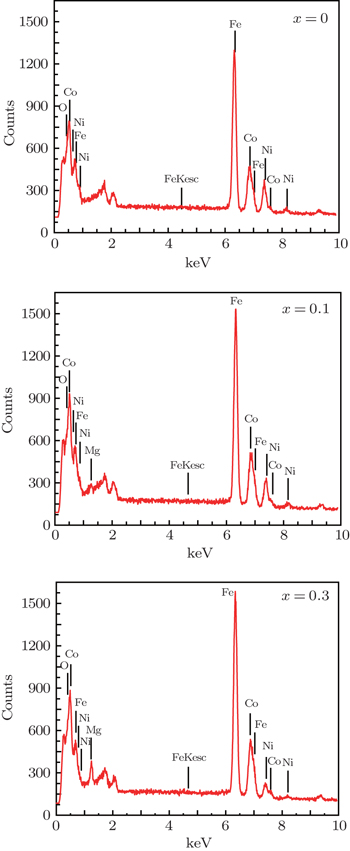 | Fig. 6. EDX patterns of Co0.5Ni0.5−xMgxFe2O4 ferrites with different compositions of Mg substitution. |
| Table 2. Compositions of ferrite samples extracted from EDX analyses. . |
The infrared spectrum provides information about the positions of ions in the crystal and the crystal vibration modes, simplifying the probing different ordering phenomena. The formation of Co0.5Ni0.5−xMgxFe2O4 is studied by Fourier transform infrared (FTIR) spectra at room temperature in a frequency range of 200 cm−1–4000 cm− 1 and the results are shown in Fig.
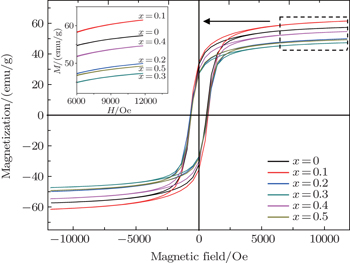
Hysteresis loops for Co0.5Ni0.5−xMgxFe2O4 (0.0 ≤ x ≤ 0.5) ferrite samples are measured in order to obtain magnetic parameters as shown in Fig.
The magnetic parameters in Table
| Table 3. Room-temperature characteristic magnetic parameters for each composition. . |
The values of magnetic moment (ηB) can be calculated from the experimental hysteresis data by using the maximum magnetizations and the molecular weights of different samples.[41] The data reported in Table
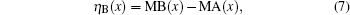
The effects of Mg content on the structural, morphological, and magnetic properties of Ni-Mg doped Co-ferrite nanoparticles are examined. The XRD patterns for all samples confirm the single phase formation of these spinel ferrites. FESEM images demonstrate that the grains are homogenously distributed and become larger as the Mg content increases and enters into the spinel lattice. The grain size is calculated using Image-J software and found to be in a range from ∼ 35 nm to ∼ 80 nm, depending on the Mg content entering into the spinel lattice. The particle sizes are small enough to be used in high-density recording media for obtaining the suitable signal-to-noise ratio. The IR curves of sintered samples exhibit absorption bands at about 595 cm−1 and 400 cm− 1, which are due to the metal–oxygen stretching vibrations of tetrahedral and octahedral sites, respectively. The hysteresis loops show the improvement in the magnetic property especially in coercivity due to the substitution of Mg ions into the spinel lattice. The squareness ratio is above 0.5 which reveals a single magnetic domain of material. Owing to all these distinguished parameters, it is affirmed that the investigated materials have high potential applications in many areas such as high-frequency devices and core materials where low eddy current losses are required.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 | |
| 39 | |
| 40 | |
| 41 | |
| 42 | |
| 43 | |
| 44 | |
| 45 | |
| 46 |