† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 11575102, 11105085, 11275116, and 11375108) and the Fundamental Research Funds of Shandong University, China (Grant No. 2015JC007).
A new strategy for the facile synthesis of very stable and mono-dispersed silver (Ag) quantum dots (QDs) is developed by laser fragmentation of bulk Ag in water using polysorbate 80 as a dispersing and stabilizing agent. The surfactant plays an important role in the formation of size-controlled Ag nano-structures. The Ag QDs have excellent photo-stability of ∼500 h and enhanced photoluminescence (PL) at 510 nm. This has significant implications for selective and ultrasensitive PL probes. Based on laser fragmentation in the biocompatible surfactant solution, our results have opened up a novel paradigm to obtain stable metal QDs directly from bulk targets. This is a breakthrough in the toxicity problems that arise from standard chemical fabrication.
Silver (Ag) nano-materials with exceptionally small nanocrystal sizes of a few nanometers including quantum dots (QDs) have received increasing attention as super-active catalysts, faster responsive sensors, biological imaging, as well as selective and ultrasensitive fluorescent probes.[1–8] This is due to their unique optical properties associated with the excitation of plasmons. Compared with large Ag nano-objects, the Ag QDs are of fine structure with a high surface area, high carrier transport-mobility, superior thermal/chemical stability, and better surface grafting properties.[4–9] Moreover, the enhanced electromagnetic field near Ag QDs can be employed in surface-enhanced Raman spectroscopy, which has opened up the possibilities of using these biocompatible Ag QDs for in vivo anatomical imaging and early stage tumor diagnosis with deep tissue penetration.[1–3] Increasing evidence has shown that the excellent properties of Ag QDs are strongly dependent on the mono-dispersed Ag QDs with exceptional stability of the colloidal solution. Meanwhile, these specific applications are often complicated because of potential toxicity issues arising from the inevitable purification procedures of nano-particles via standard chemical fabrication based on the reduction of a precursor salt with a reducing agent. The development of new methods of conveniently synthesizing stable and mono-dispersed Ag QDs is important.
Laser fabrication in liquid is a new green approach to the synthesis of novel meta-stable phases of materials.[10–16] It is an attractive technique with a high non-equilibrium processing character. It offers a breakthrough in toxicity challenges. Stable Ag nano-particles and Ag2O/Ag nano-colloids have been reported by using laser ablation in different surfactant solutions.[15–17] Yan et al. fabricated a mixture of Ag2O/Ag nano-cubes, pyramids, triangular plates pentagonal rods, and bars with sizes ranging from 400 nm to 1000 nm by laser ablation of Ag in polysorbate 80, 40, and 20 aqueous solutions.[16] Stable Ag nano-colloids with diameters from ∼10 nm to ∼30 nm have been generated by laser ablation in isopropanol, ethylene glycol, and biopolymers such as chitosan.[17] By using dodecanethiol solution in heptanes, Ag nano-particles with the diameters of 5 nm–30 nm were produced by laser ablation.[15] It should be noted that these reports mainly focused on the synthesis of Ag nano-particles with large sizes or the fabrication of smaller Ag nano-colloids via some potentially toxic surfactants such as dodecanethiol, isopropanol, heptanes, etc. The schemes, which are environmentally friendly or have negligible toxicity, make stable and small Ag QDs remain incomplete.
Herein, we design a simple, versatile, and rapid route to controllably synthesize stable and mono-dispersed Ag QDs directly from a bulk Ag target based on laser fragmentation in liquid solution containing distilled water and polysorbate 80. The polysorbate 80 is a dispersing and stabilizing agent and is a nonionic surfactant or biocompatible emulsifier. It is often used as an emulsifier in foods or a solubilizer in mouthwash or an effective drug delivery vehicle in the brain, etc.[16] Moreover, the liquid can be adjusted to obtain desirable nano-structures because the polysorbate 80 concentration plays a critical role in the synthesis of nano-scale Ag nano-particles during laser ablation of a Ag target.
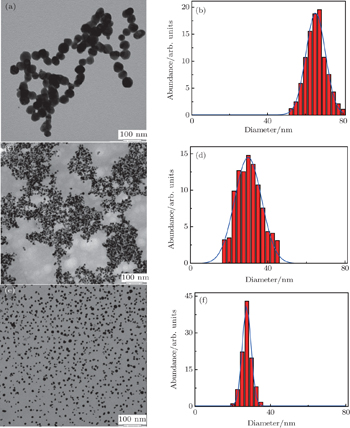
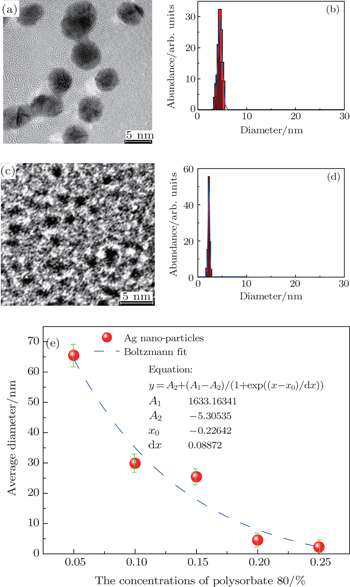
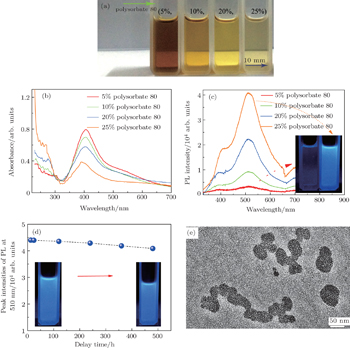
We use polysorbate 80 concentrations ranging from 0% to 25% v/v, and the mean diameters of the Ag nano-particles substantially decrease from about 65.5 nm to 2.2 nm resulting in the photoluminescence (PL) spectra of Ag nano-clusters at 510 nm that is drastically enhanced from about 3438 a.u. to ∼40618 a.u. (The unit a.u. is short for atomic unit.) Moreover, the strong PL emission of Ag QDs shows excellent photo-stability after keeping them in liquid for 500 h. Meanwhile, a detailed discussion of the relevant mechanism is addressed. The aim of this work is to extend a new method of synthesizing the highly photoluminescent and stable Ag QDs. We then use this approach to generate other metal complex nano-structures by laser fragmentation.
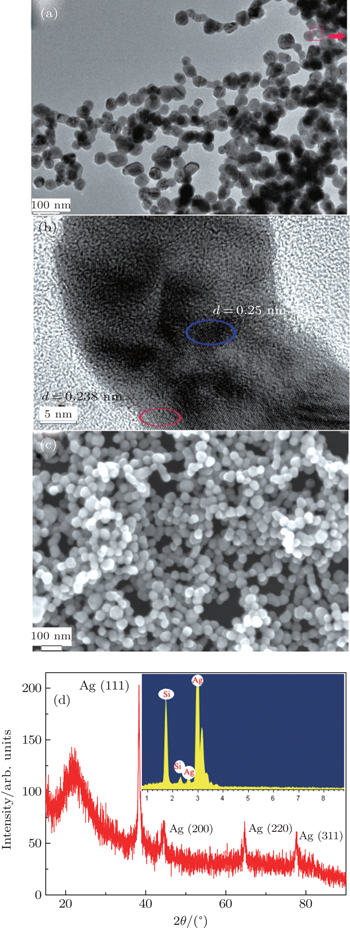
This scheme used pulsed laser ablation of a solid target in liquid. It is similar to that described in previous studies.[10–12,14–17] In a typical experiment, a well-polished pure (99.99%) Ag target was placed on the bottom of a rotating glass dish with a speed of ∼100 rpm that was filled with polysorbate 80 and distilled water to 3 mL (Vpolysorbate : Vwater = 0% v/v–25% v/v). A Q-switched Nd-YAG (yttrium aluminum garnet) laser (Quanta Ray, Spectra Physics) beam operating at a wavelength of 1064 nm with a pulse duration of 10 ns and 5 Hz repetition rate was focused onto the Ag target surface. The laser beam was focused on the target by a quartz lens with 50-mm focal length, and the average spot size of the laser beam at the target was measured to be about 300 μm. During the laser irradiation, the liquid was protected by N2 gas at a flow rate of 10 sccm to avoid the formation of surface oxidation Ag nano-particles in the liquid. The laser beam intensity was about 6.5 GW/cm2, and the ablation lasted 1.5 h. After laser fragmentation, the Ag nano-particles were repeatedly centrifuged at 18000 rpm for 20 min in an ultracentrifuge, and the precipitates were carefully washed in ethanol three times. The sediments were dropped on a copper mesh and dried in an oven at room temperature for observation by transmission electron microscopy (JEOL-JEM-2100F). In addition, the crystallographic investigations of the Ag nano-particles were analyzed by x-ray diffraction (XRD) patterns (Rigaku RINT-2500VHF) using Cu Kα radiation (λ = 0.15406 nm). Morphological investigations and chemical composition measurements were performed by field emission scanning electron microscope (SEM, Hitachi S-4800) equipped with energy-dispersive x-ray spectroscopy (EDS). The absorption spectra of the Ag colloidal suspensions were measured with a UV–Vis–IR spectrometer (Cary 50). A 325-nm CW semiconductor laser (18 mW) was used as an excitation source with a spot size of ∼170 μ m. The PL spectra of the Ag colloidal suspensions were dispersed by a monochromator (Jobin-Yven iHR320) with a 300/mm grating, and detected by a thermoelectrically cooled CCD detector (Synapse).
After cumulative pulse laser ablation of pure Ag metal in distilled water with the polysorbate 80 concentration of 0% v/v, low magnification and high resolution images of the nano-particles are collected with transmission electron microscopy (TEM; Figs.
The above results in Fig.
To verify this laser fragmentation process, we increase the concentration of polysorbate 80 via water to 5%, 10%, and 15% v/v, respectively. The typical TEM images and the corresponding particle size distribution histograms of the final particles are illustrated in Figs.
Finally, the unique optical properties of size-controlled Ag nano-particles are illustrated by the absorption and PL spectra (Fig.
In this work, size-controlled Ag nano-structures are carefully devised through multiple pulse laser fragmentation of a Ag metal target in a liquid medium containing nonionic surfactant polysorbate 80 as a dispersing and stabilizing agent. The well mono-dispersed Ag QDs with a mean size of approximately 2 nm can be obtained by simply increasing the surfactant concentration in distilled water to 25% v/v. In addition to the enhanced PL emission in the visible region, the remarkable photo-stability of these water-dispersed Ag QDs remains for about 500 h after synthesis. The product has negligible toxicity by using cell proliferation. This is because there are no toxic elements used during fabrication. This work confirms the possibility of using these Ag QDs for in vivo anatomical and early stage tumor diagnosis if the PL emission can be shifted to the infrared region.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 |