† Corresponding author. E-mail:
‡ Corresponding author. E-mail:
Project supported by the Joint Funds of the National Natural Science Foundation of China (Grant No. U1530402), the National Natural Science Foundation of China (Grant No. 11004163), the Fundamental Research Funds for the Central Universities, China (Grant No. 2682014ZT31), the Department of Energy National Nuclear Security Administration (Grant No. DE-NA0001974), and the Department of Energy Basic Energy Sciences (Grant Nos. DE-FG02-99ER45775 and DE-AC02-06CH11357).
Two kinds of glassy sulfurs are synthesized by the rapid compression method from liquid sulfur at temperatures below and above the λ -transition point. The glassy sulfur has different colors and transparencies, depending on temperature, which may inherit some structural information from the λ -transition. Raman spectrum studies of these samples show that a large fraction of polymeric chains exist in the glassy sulfur, even in the one solidified from T < Tλ. We find that a higher compression rate instead of a higher temperature of the parent liquid captures more polymeric chains. Pressure-induced glassy sulfur presents high thermal stability compared with temperature quenched glassy sulfur and could transform into liquid sulfur directly without crystallization through an abnormal exothermic melting course. High energy x-ray diffraction is utilized to study the local order of the pressure-induced glassy sulfur.
At 159 °C a phase transition in the liquid sulfur is known as the λ -transition, with an abnormal increase of viscosity with increasing temperature over a temperature range of 20 °C, accompanied by rapid changes in optical and thermodynamic properties.[1–3] The λ -transition has attracted considerable experimental as well as theoretical attention for many decades.[3–8] However, no model satisfactorily accounts for its puzzling temperature dependence of viscosity. The λ-transition is explained mostly as a liquid-liquid transition from an S8 ring monomer to a polymeric phase.[3,4] The presence of polymeric sulfur is the key factor for extracting information about many thermodynamic aspects of the λ-transition.[8] In order to study the liquid structures at different temperatures, one can quench molten sulfur from various temperatures to low temperature (for example, to liquid nitrogen temperature), and study the local structure of quenched glassy sulfur.[8–11] Although these studies demonstrate that there is an obvious content increase of polymeric chains after the λ-transition, the formation of a glass composed of pure S8 ring molecules, quenched from T < Tλ, has not yet been reported.[8] The investigation of the λ-transition which has been tackled for more than 150 years is still an attractive challenge.[6,7,12]
As is well known, the quenched glassy sulfur is very unstable. Slightly above the glass transition temperature Tg, say, in a range of −40 °C–−20 °C depending on thermal history, gradual crystallization takes place.[4,8,13] At room temperature, the crystallization can be completed very quickly.[4,8] In the crystallization process, the polymeric chains revert back to the more stable S8 rings. The extremely high tendency towards crystallization precludes the quenched glassy states from being characterized in detail, which restricts our understanding of the λ-transition.[4] Recently, a pressure-induced solidification of glassy sulfur from liquid sulfur was developed, which demonstrated an extraordinary thermo-dynamical stability.[14–16] When heated to above room temperature, crystallization could be effectively avoided by using at least a 10 K/min heating rate and polymeric fraction remains until melting.[17,18] The high thermal stability allows us to study the λ-transition by characterizing the glassy sulfurs solidified from different liquid temperatures. In this work, we prepare two kinds of glassy sulfurs from liquid sulfur below and above the λ-transition and with two different compression rates. We examine the relative fraction of the polymeric content by exploiting the distinctive Raman signals at 472 cm−1 for S8 rings and 461 cm−1 for polymeric chains respectively.[13] The pressure dependence of the polymer content, to which the liquid is rapidly compressed, and the mechanism of high thermal stability of the pressure-induced glassy sulfur are also discussed.
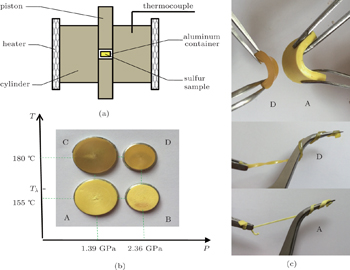
Figure
The colors of the above mentioned four samples inherit the information from sulfur λ-transition information. It is known that the color of liquid sulfur changes from light-yellow to dark yellow during λ-transition and finally dark-red at higher temperatures. These four samples were further analyzed by x-ray diffraction (XRD) (X’ Pert. PRO. MPD. Philips) by using Cu Kα radiation. Raman spectroscopy (in-Via, Renishaw) with a 532-nm excitation laser with a spectral resolution of 1 cm−1 was preformed. The calorimetric measurement of melting behaviors was conducted on the TA-2920 instrument with a heating rate of 10 K/min. The pair distribution function studies of A and D samples were conducted with energy-dispersive x-ray diffraction (EDXRD) mode using a VX-3 Paris-Edinburgh press at the white x-ray beamline 16-BM-B, High Pressure Collaborative Access Team (HPCAT) at the Advanced Photon Source, Argonne National Laboratory. The incident white x-ray beam was collimated to a size of 0.3 mm (vert.) ×0.1 mm (horiz.) by using two sets of tungsten slits. The measurements were conducted at 233 K for sample A and 235 K for sample D under ambient pressure. The XRD patterns were collected by a Ge solid-state detector at nine different 2θ angles (3°, 4°, 5°, 7°, 9°, 12°, 16°, 22°, and 28°), to cover a large Q range (Q = 4πE sinθ/12.398, where E is the x-ray energy up to 125 keV). The typical time for collecting one set of diffraction patterns of nine angles was about 2 h. The details of the EDXRD measurement and data analysis method are described in Ref. [20].
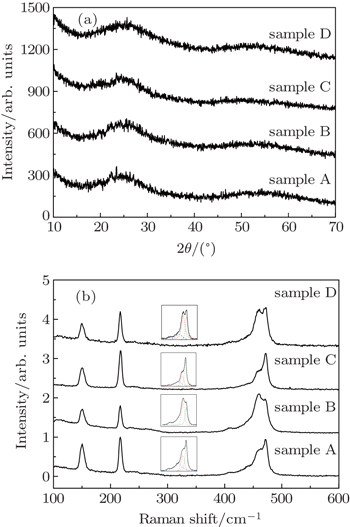
Figure
The relative weight fraction of the polymeric content in glassy sulfur is a key parameter. If the λ-transition is essentially a living polymerization transition, the liquid sulfur at T < Tλ should consist of S8 ring molecules and correspondingly the glassy sulfur composed of pure S8 ring molecules should be available. However, the formation of a glass composed of pure S8 ring molecules, rapidly quenched from T < Tλ, has not yet been reported.[8] In the present work, we measure the fractions of the polymeric content in glassy sulfurs, solidified from T < Tλ and T > Tλ by rapid compression. The central issue is to check whether a glassy sulfur composed of pure S8 ring molecules is possible. Quenched glassy sulfur referred to as ‘elastic sulfur’ displays good elasticity like a typical rubber.[4] Its elastic property is attributed to polymeric chains frozen in the glassy sulfur.[4] In this work, four samples A, B, C, and D all display good elasticities, which implies that the polymeric chains may exist in the glassy sulfur solidified from T < Tλ. The existence of polymeric chains is testified by Raman spectrum analysis. Figure
Then the next question would be whether the polymeric chains are inherited from the parent liquid or formed during the rapid compression. This issue can be deduced from the percentage change of relative Raman vibration intensity from S8 ring (472 cm−1) to a polymeric chain (461 cm−1) across the λ-transition and compression pressure. Considering the peak areas at 472 cm−1 and 461 cm−1 as the corresponding weight factors for the S8 ring and polymeric chain, the ratio A461/A472 provides a reasonable finger print on the ring-chain transition. For samples A, B, C, and D, the ratios are 0.66, 1.33, 0.46, and 1.81, respectively. There is not a clear trend by comparing the temperature effects between A and C (decreasing), and between B and D (increasing), but the pressure effect is pretty consistent and significant (for samples A and B, their A461/A472 ratios change from 0.66 to 1.33, and for samples C and D, the ratios change from 0.46 to 1.81). It is clear that a higher compression rate and a larger amplitude of pressure jump, instead of increasing the temperature of the parent liquid, could capture more polymeric chains. So, the polymeric chains are properly formed in rapid compression course, and a glass composed of pure S8 ring molecules cannot be formed even when solidified from T < Tλ. Furthermore, we should remember that the glassy sulfurs solidified from below and above the λ-transition show different colors and transparencies, which is consistent with liquid sulfur. Does this mean that the two sets of glassy sulfurs inherit some information of λ-transition? But the existence of polymeric chains in both sets of glassy sulfurs suggests that the polymeric chains are not the factor that leads to the color distinction. In the future, the analyses of the two sets of glassy sulfurs will be conducted to reveal more information under the λ-transition. We also notice that the vibration mode located at 461 cm−1 gains intensity at the expense of the 472 cm−1, 220 cm−1, and 150 cm−1 modes. The competition between the S8 rings and polymeric chains indicates that joining which kind of cluster is an alternative choice for the sulfur atoms. The mechanism of forming polymeric chains needs further study by in-situ investigations or simulations in the future.
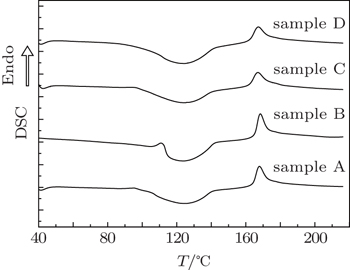
As mentioned earlier, the pressure-induced glassy sulfur shows extraordinary thermo-dynamical stability.[14–16,21] When heated to above room temperature, crystallization could be effectively avoided by using at least 10 K/min heating rate and polymeric fraction remains until melting.[17,18] We reported a direct transition from pressure-induced glassy sulfur to liquid accompanying an abnormal exothermic effect.[17] The exothermic transition from glassy sulfur to liquid sulfur likely possesses an additional exothermic contribution stemming from the polymeric chain-S8 ring transition, which affects its change of internal energy and makes it much larger than that of the endothermic process.[18] Since the large fraction of polymeric chains exist in samples A, B, C, and D in this work, the extraordinary exothermic melting should occur in all four samples. Figure
In order to elucidate the structural characteristics of the glassy sulfur solidified by the rapid compression method, we conduct a high energy x-ray diffraction (XRD) experiment at High Pressure Collaborative Access Team (HPCAT). Figure
In this work, we solidify glassy sulfurs from liquid sulfur at temperatures below and above the λ-transition point by a rapid compression method. A large fraction of polymeric chains exist in the glassy sulfur that is solidified at T < Tλ. The glassy sulfur composed of only pure S8 ring molecules is not detected by using the rapid compression method. We find that a higher compression rate and larger amplitude of pressure jump, instead of a higher temperature of the parent liquid, can capture more polymeric chains. The polymeric chains are properly formed in the rapid compression course. The glassy sulfurs solidified from below and above the λ-transition show different colors and transparencies, which is consistent with liquid sulfur and it shows us that the two kinds of glassy sulfurs may inherit some information about the λ-transition. But the existence of polymeric chains in both sets of glassy sulfur suggests that the factor that causes color distinctions does not refer to polymeric chains. In the future, the analyses of the two sets of glassy sulfurs will be conducted to reveal more information under the λ-transition.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 |