† Corresponding author. E-mail:
Project supported by the Natural Science Foundation of Fujian Province, China (Grant No. 2014J01025), the National Natural Science Foundation of China (Grant No. 11275261), the Natural Science Foundation of Guangdong Province, China (Grant No. 2015A030313005), and the Fund from the Fujian Provincial Key Laboratory for Plasma and Magnetic Resonance, China.
A stable and homogeneous well-aligned air microplasma device for application at atmospheric pressure is designed and its electrical and optical characteristics are investigated. Current-voltage measurements and intensified charge coupled device (ICCD) images show that the well-aligned air microplasma device is able to generate a large-area and homogeneous discharge at the applied voltages ranging from 12 kV to 14 kV, with a repetition frequency of 5 kHz, which is attributed to the diffusion effect of plasma on dielectric surface. Moreover, this well-aligned microplasma device may result in the uniform and large-area surface modification of heat-sensitive PET polymers without damage, such as optimization in hydrophobicity and biocompatibility. In the biomedical field, the utility of this well-aligned microplasma device is further testified. It proves to be very efficient for the large-area and uniform inactivation of E. coli cells with a density of 103/cm2 on LB agar plate culture medium, and inactivation efficiency can reach up to 99% for 2-min treatment.
Atmospheric pressure cold microplasma has recently attracted much attention especially in biomedical application (plasma sterilization,[1] living tissue treatment,[2] induction of apoptosis,[3] cancer therapy[4]) and material processing (surface modification,[5,6] processing,[7] nanotech application,[8]) due to its high plasma density, low gas temperature, high discharge stability, and the simplicity of the experimental set-up. However, in order to obtain stable microplasmas at atmospheric pressure, the feed gases used for most plasma devices are noble gases, such as helium or argon, which adds to the difficulties for widespread application. Briefly, it is difficult to generate a stable and homogeneous air discharge at atmospheric pressure due to the high breakdown voltage or the nonhomogeneity of filamentary discharges.[9–13]
However, the utilization of atmospheric air can greatly reduce the complexity of the device and enhance the production of reactive species, mainly including (i) negative particles (e−, O−, and 
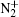

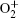
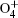
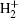
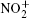
The small size of the atmospheric pressure air microplasma also prevents it from being used in some areas such as plasma inactivation and decontamination of reusable medical tools.[1,17] Several researches are under way to overcome this difficulty. Eden et al. pointed out that microcavity plasmas had advanced rapidly in surpassing several milestones, primarily with respect to electron density and cavity geometries, and some new avenues of research with noble gases, such as Ne, Xe or gas mixture, at a total pressure of several hundred Torrs were established.[18] For this purpose, our research team has conceived another effective approach and contrived a novel well-aligned microplasma array.
The microplasma device consists of 6×6 well-aligned, stable microplasma jets formed near the ends of hollow optical fibers sealed at one end at room temperature. The unique advantage of this device is that these microplasma jets with the polarity effect of discharge can aggregate along the surface of dielectric and form a large-area uniform surface plasma. The detailed schematic of this plasma device is illustrated below, and its electrical and optical characteristics are analyzed based on experimental data. Moreover, as a case of application, this well-aligned air plasma is used for large-area uniform surface modification of polyethylene terephthalate (PET) polymers and plasma inactivation of E. coli cells at room temperature.
The schematic diagram of the microplasma array is shown in Fig.
Applied voltage and discharge current are simultaneously measured by using a Tektronix DPO5024 digital oscilloscope with a 1:1000 H.V. probe (Tektronix P6015A) and a Tektronix P2220 current probe. Measurements for the charges across this capacitor and the applied voltage across the discharge device result in a Lissajous figure, which is used to calculate the discharge power.[19,20] Contact angle measurements are performed by using the sessile drop method with a contact angle goniometer (Phoenix 300, SEO) at an ambient temperature after 5.0-μl water drops have been applied to the surface of samples. Contact angle measurements of the treated polymers immediately follow plasma treatment with a delay of 3 min–5 min. For each sample, at least three drops are placed at different locations on the large-area polymer. Surface chemical compositions of PET polymers are analyzed by XPS. These analyses are performed on a Physical Electronics PE5800 ESCA/Auger electron spectroscopy system. Survey scans (0 eV–1000 eV), acquired with an analyzer pass energy of 93.9 eV, are used to determine elemental compositions. A photoelectron take-off angle of 45° is used for all spectra. High-resolution C1s spectra are deconvoluted to analyze the C bonding environment at the surfaces of treated polymers.
Photoelectron multiplier tubes (PMTs) (Hamamatsu CR131) are used to study the optical emission from the well-aligned microplasmas, placed at 25 mm away from the broadside of discharge area. The output of the PMTs, the voltage and current waveforms are simultaneously recorded by Tektronix DPO 5024. In the front part of PMT, there are two slits each with 1 mm in width and 20 mm in thickness, and they are separated by a distance of 100 mm. In addition, an ICCD camera (ANDOR, iStar DH712) is fixed at 50 cm above the discharge region to capture the discharge propagation processes. The ICCD camera is triggered by a square wave with a peak value of 2.6 V, which is generated by the digital delay generator (DG645). Also, the digital delay signals are recorded simultaneously by using Tektronix DPO5024 oscilloscope. Optical emission spectra from the discharge region are obtained by using an Andor SR-750i grating monochromator (grating groove at 2400 lines/mm and glancing wavelength at 300 nm). After the diffraction of the grating, the output light signal is converted into digital signals by CCD camera and stored in the connected computer.
During the material modification, the 200-μm-thick PET (Teijin company, Japan) sample is placed on the quartz plate. The PET sample with a melting point of 254 °C is amorphous. The plasma device is fixed at 1 mm above the sample for performing this plasma treatment. The adhesion of blood platelets on untreated or plasma-treated PET polymers is investigated and evaluated by using SEM (Hitachi S4800). The platelet-rich blood plasma from a healthy donor is diluted 10 times with pure water. After being incubated for 2 h, the sample is washed by phosphate buffered saline (PBS) several times to remove loosely-clung platelets. The PET sample is then exposed to 25% glutaraldehyde for 2 h for fixation, dehydrated with alcohol, then dealcoholizated with isoamyl acetate solution and subsequently dried under ambient conditions.
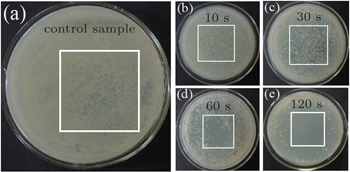
The E. coli cells with a density of 103/cm2 on LB agar plate culture medium are used to investigate the inactivation efficiency of the visually large-area uniform discharge. A single colony of E. Coli is inoculated into 200 ml of LB liquid medium (Tryptone: 2 g, Yeast Extract: 1 g, distilled water: 200 g, pH = 7.2) and is cultivated on the shaking table with a rotation speed of 150 rpm at 28 °C. For plasma inactivation treatments, 100 μl of the E. coli culture is deposited on the LB agar plate culture medium to make a sample have a density of 103/cm2. During the bacterial treatment, the petri-dish containing E. coli cells is grounded. The plasma device is fixed at 1 mm above the sample for performing this plasma treatment. The treatment time varies from 10 s to 2.0 min. For all inactivation and modification treatments, the air flow rate and VP are kept to be 4 SLM and 14 kV respectively. Likewise, for the plasma-untreated (control) sample cultivation, the 4 SLM air feed gas is added into the well-aligned plasma device, and kept for 2 min for comparison. Following this, the plasma-treated culture media containing E. coli cells are incubated for 24 h in a 37-°C thermostatic container for observation while the untreated one is used as a control sample. Afterward, cellular colonies are counted to calculate the inactivation efficiency in the treated area. The detailed process is similar to that reported in our previous work.[21] All the experiments reported are repeated three times, and all the data obtained under the same experimental condition are repeatable and consistent.
Figures
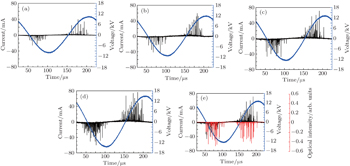
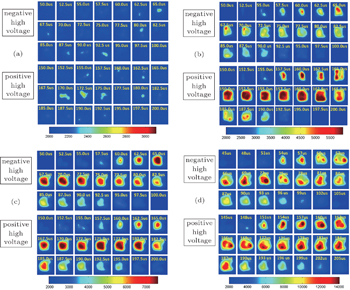
To investigate the discharge characteristics of the well-aligned microplasma array, the high-speed ICCD camera is used to capture the dynamics of the air discharge. Figure
Figure
Figure
The ICCD images display that a stable and large area diffusion discharge could be achieved in a wide range of discharge voltage. As the discharge voltage increases, the plasma area is significantly enlarged both horizontally and vertically (see Fig.
To overcome the discharge excitation threshold requires the applied voltage Ua to be increased to reach the breakdown voltage Ub over the discharge gap d. Ub is only related to the kind of gas used and the discharge gap d, and can be considered as a constant in this aligned air plasma discharge. Then Ua is given by
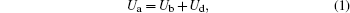
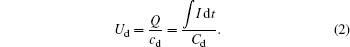
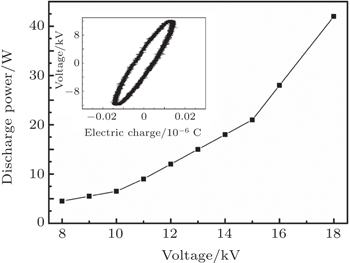
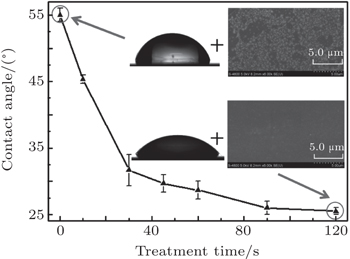
Such a diffusing and large-area microplasma with low temperature is highly suitable for large-area surface modification of materials or biological sterilization. The plasma has no detrimental effect on the heat-sensitive material due to its low gas temperature, which is indicated by the discharge power from Lissajous figures; the discharge power is calculated to be about 16 W. The sessile water-drop contact angles of PET samples are plotted as a function of the plasma treatment time as shown in Fig.
Plasma treatment may result in a hydrophobic surface of PET sample and have a great effect on the contact angle of PET sample. The contact angle of PET sample decreases markedly from (55.0±1.0)° to (25.5±0.5)° with the plasma treatment time being 2 mins. Figure
The obvious differences in contact angle and platelet adhesion between untreated and plasma-activated PET samples are attributed to their surface change in chemical composition and bonding environment. Survey-scan XPS spectra show that carbon, nitrogen and oxygen are existent, and they are the built blocks of the film. Figure
Figure
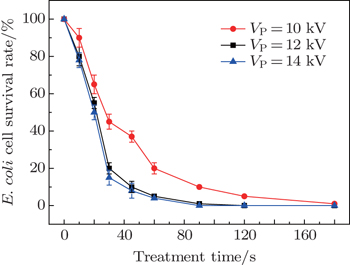 | Fig. 8. Plots of survival rate of E. coli versus processing time, obtained at various applied voltages. |
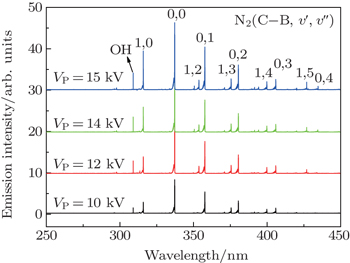
The optical emission spectra (OES) with a wavelength range from 200 nm to 500 nm at various discharge voltages are collected as shown in Fig.
In this paper, the well-aligned microplasma operating at room temperature is designed for the large-area surface modification of heat-sensitive PET polymers and inactivation of resistant E. coli cells. This design can generate a kind of visible large-area and homogeneous discharge, resulting in a uniform surface interaction of plasma species with PET polymers and E. coli cells. It is suggested that various plasma-activated particles including charged species, UV photons and radicals, which are natural carriers of energy, play the leading role in the plasma modification and inactivation process, provided they excite a polymer, or break the chemical bonds at the polymer surface or directly reach E. coli cells.
The surface interactions of both radicals and energetic ions with the PET surface can create the dangling bonds and contribute to the incorporation of oxygen and nitrogen.[32] Our SEM measurements also show that the surface topography of the PET sample is not influenced by the plasma treatment. The temperature of the plasma-treated PET sample is measured with an infrared radiation thermometer, and it is usually lower than 40 °C. Therefore, the stable and homogeneous air plasma operating at room temperature does not cause damage to the heat-sensitive polymers and is suitable for the large-area surface modification of polymers in biomedical application.
Charged species are also supposed to induce the rupture of the outer membrane of bacteria cells.[33,34] The electrostatic force caused by charge accumulation on the outer surface of the cell membrane can overcome the tensile of the membrane and cause its rupture. Metastable or excited species can emit UV photons for plasma inactivation. The UV radiation with doses of several milliwatts per cm2 may cause lethal damage to cells.[35] However, no UV radiation in the 200 nm–300 nm wavelength range is observed by our OES measurement. It is generally believed that UV photons do not play a major role in the inactivation process by atmospheric-pressure cold plasma.[22,33,34] The O radicals are believed to play a crucial role in the plasma inactivation of microorganism.[35,36] Moreover, the frequent collisions between O radicals and O2 molecules can result in the production of ozone (O + O2 + M → O3).[4] Unsaturated fatty acids and sulfhydryl groups are readily oxidized with ozone. These oxidization process may lead to the inactivation of microorganisms and bacterial spores. The presence of these plasma-activated species can therefore compromise the function of the cell membrane which serves as a barrier against the transports of ions and polar compounds into and out of the cells.
The well-aligned microplasma device designed in this study is successfully utilized to generate a large-area and homogeneous plasma at atmospheric pressure in air. The surface charges from microplasma jet units may form the electric field along the quartz surface, and tend to be distributed and diffuse uniformly at an applied voltage in the range of 12 kV–14 kV, which leads to the uniform and large-area surface discharge for modifying the heat-sensitive PET polymers and inactivation of E. coli cells. This technique demonstrates its potential for surface inactivation in biomedical application, as well as large-area surface modification in industrial application.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 |