† Corresponding author. E-mail:
We investigate whether AlCl and AlBr are promising candidates for laser cooling. We report new ab initio calculations on the ground state X1Σ+ and two low-lying states (A1Π and a3Π) of AlCl and AlBr. The calculated spectroscopic constants show good agreement with available theoretical and experimental results. We also obtain the permanent dipole moments (PDMs) curve at multi-reference configuration interaction (MRCI) level of theory. The transition properties of A1Π and a3Π states are predicted, including the transition dipole moments (TDMs), Franck–Condon factors (FCFs), radiative times and radiative width. The calculated radiative lifetimes are of the order of a nanosecond, implying that they are sufficiently short for rapid laser cooling. Both AlCl and AlBr have highly diagonally distributed FCFs which are crucial requirement for molecular laser cooling. The results demonstrate the feasibility of laser cooling AlCl and AlBr, and we propose laser cooling schemes for AlCl and AlBr.
Ultracold molecules offer a unique opportunity for studying chemical reactions at the quantum state level. Recently, the laser cooling of diatomic molecules has been demonstrated in the laboratory.[1–3] It is notable that molecule SrF1 has been directly cooled by Doppler and Sisyphus cooling for the first time. Later YO2 and CaF3 have also been experimentally cooled by laser cooling. It would be good to note that similar schemes have now been proposed for molecular ions (eg the molecular hydrogen ions[4] HD+). The researches on the laser cooling in our country are also motivated by all sorts of prospective applications.[5–7] Since there are more than 90 elements in the periodic table, it may form more than 4000 different diatomic molecules. Naturally the search for potential laser cooling candidates is getting people to pay attention. For laser cooling of a molecule, it is very difficult to keep a closed optical pumping cycle with repeated optical spontaneous emissions due to the molecule’s complex internal structure. SrF, YO, and CaF all demonstrate highly-diagonal Franck–Condon factors (FCFs) which suppress decays to unwanted sublevels. So in order to be a possible source for laser cooling, the molecule must meet the first criteria: highly-diagonal FCFs. The highly-diagonal FCFs would limit the number of lasers required to keep the molecule in a closed-loop cooling cycle. A second criteria is that rapid laser cooling calls for shorter lifetimes. SrF, YO, and CaF all have short lifetimes. The calculations of FCFs and lifetimes would help us to identify promising candidates for laser cooling theoretically. Thus, we use ab initio calculations to investigate the possibility of laser cooling AlCl and AlBr in this paper.
In 2004, a brief survey of laser cooling candidates was presented by Di Rosa.[8] The list of candidates included aluminum compounds. The laser cooling of the related diatomics AlH and AlF has been discussed in detail by Wells and Lane.[9] They identified AlH and AlF as suitable laser cooling candidates. Now we would like to choose the two molecules AlCl and AlBr, since they have large emission probability (thus high scattering rates), very low scattering probabilities into off-diagonal bands 0 → 1 and 0 → 2, the desirable 1Π ← 1Σ+ cycling transitions and extremely short excited state lifetimes.
Aluminum monohalides (AlCl and AlBr) are readily produced in the vapor phase by high temperature pyrolysis[10] and from chemiluminescent reactions[11] of Al atoms with the halogens. A high-resolution emission spectrum of AlCl at 20 μm was conducted by Hedderich et al.[12] They have found that the ground state X1Σ+ of AlCl possesses a deep potential. The previous experimental studies of electronic excited states for AlCl consisted mainly of absorption and emission spectra from the first singlet–singlet (i.e. A–X), triplet–triplet (b–a) and triplet–singlet (a–X) systems.[12–18] Theoretical calculations have also been performed for AlCl over the past several decades. For AlCl, the potential curves for the X1Σ+ and A1Π states, and A1Π vibrational lifetimes were given by Langhoff et al.[19] Brites et al.[20] obtained the lifetime of AlCl for the A1Π ν′ = 0 level of 5.9 ns. These authors all agreed that AlCl has highly-diagonal FCFs and short radiative lifetimes. Several experimental and theoretical studies of the AlBr molecule are also available. Bredohl et al.[21] have provided an extensive high-resolution band-by-band analysis of 28 bands of the A–X transition of AlBr. The A1Π –X1Σ+ transition of AlBr was recorded by Fleming et al.[22] using a Bruker IFS 120 HR Fourier transform spectrometer. Their results showed that the 0–0 band was the most intense and the Δν = 0 sequence dominated the observed spectrum. Recent theoretical calculations have been performed for 12 electronic states for AlBr by Hamade et al.[23] The radiative lifetime for the ν′ = 0 level of the A1Π state was computed by Langhoff et al.[19] to be about 8.5 ns for AlBr using averaged complete active space self-consistent field (CASSCF)[24,25] calculations.
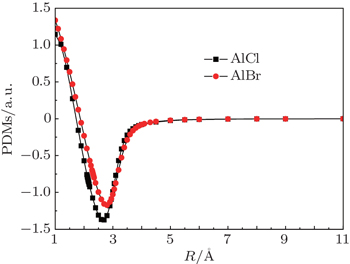
As stated above, AlCl and AlBr have been researched systematically. Nevertheless, systematic studies of laser cooling of AlCl and AlBr are not available in the literature. So our work focuses on identifying whether AlCl and AlBr are possible candidates for laser cooling with ab initio calculations. Because AlCl and AlBr both possess 1Π ← 1Σ+ transition, the FCFs, transition dipole moments (TDMs), radiative lifetimes and radiative widths of the A1Π –X1Σ+ transtions of AlCl and AlBr are predicted. Also accurate determinations of the spectroscopic constants, potential energy curves (PECs) and permanent dipole moments (PDMs) of the X1Σ+ and A1Π states of AlCl and AlBr have been given. Additionally, there is a low lying a3Π state between the A1Π and ground states for AlCl and AlBr. The PECs, spectroscopic constants of a3Π state, and the transition properties of the a3Π state to the ground state are also obtained. Finally, we briefly design laser cooling schemes for AlCl and AlBr.
The present paper is organized as follows. In Section 2 we give the details of our calculations. Section 3 presents the results and discussion, outlining laser cooling schemes for AlCl and AlBr. The conclusions are summarized in Section 4.
All our ab initio calculations are performed using the MOLPRO package.[26] The ground state (X1Σ+) and two low-lying excited states (A1Π and a3Π) for AlCl and AlBr have been conducted with multi-reference configuration interaction (MRCI) plus Davidson corrections[27–29] which is based on CASSCF[24,25] wave functions. For AlBr, the scalar relativistic effects are taken into account using the Douglas–Kroll–Hess[30,31] transformation of the relativistic Hamiltonian. The spectroscopic constants (Re, De, we, weχe, Be, Te) are derived by using Le Roy’s LEVEL 8.0 program.[32] The PDMs and TDMs of AlCl and AlBr are evaluated by taking the expectation and transition values using the MRCI wave functions. Spin–orbit coupling is ignored in our calculations, since the principal transitions under investigation are singlet states.
In our calculations, the basis sets aug-cc-PVQZ[33] are used for Al and Cl. As for Br, we choose the small-core scalar relativistic effective core potential ECP10MDF[34] together with the corresponding valence basis sets.[35] Owing to the limitation of the MOLPRO software package, C2ν point group symmetry has been considered for AlCl and AlBr in all computations, which holds (a1, b1, b2, a2) irreducible representations. For AlCl, eight molecule obitals (MOs) are put into active space, including four a1, two b1, two b2 symmetry MOs (4220), which correspond to the 3s3p shells of the Al atom and 3s3p shells of the Cl atom. The active space of AlBr consists of eight MOs 4a1, 2b1, 2b2, 0a2 (4220) which correspond to Al 3s3p and Br 4s4p.
Figure
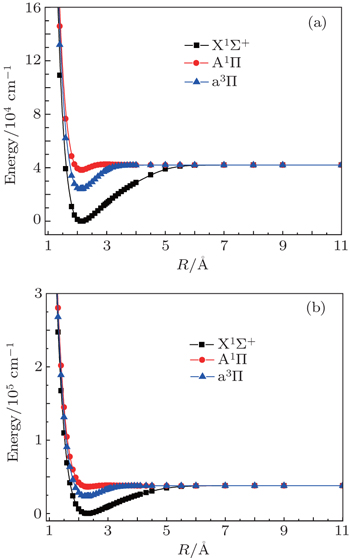 | Fig. 1. Potential energy curves of the first three electronic states of AlCl (a) and AlBr (b) at the MRCI level of theory. |
| Table 1. Calculated spectroscopic constants of X1Σ+, A1Π, and a3Π states of AlCl and AlBr at the MRCI level of theory. . |
For AlCl, our results are close to the experimental data. The percentage error in Re, we, and weχe are 0.70%, 0.69%, and 5.80% for the ground state X1Σ+ of AlCl. Findings for the excited state a3Π of AlCl are similar; the percentage error in Re, we, and weχe are 0.57%, 0.19%, and 19.72% compared with the experimental data.[17] Although the deviation of the weχe seems to be a little large, our calculated values are closer to the experimental data[17,35] than the theoretical results obtained by Brites et al.[20] The percentage error in weχe calculated by Brites et al.[20] are 212.56% and 76.09% for the X1Σ+ and a3Π states of AlCl. The computed ground state dissociation energy De of 5.22 eV compares well with the experimental value of (5.25±0.01) eV.[14,36] Our calculated Te result is only 49 cm−1 larger than the observed data[37] for the A1Π state of AlCl.
We turn now to the discussion of AlBr. Our calculated spectroscopic constants of AlBr are also close to the experimental values, compared with previous theoretical data obtained by Langhoff et al.[19] The calculated Re, we, and Be for X1Σ+ state of AlBr are 2.306 Å, 378.72 cm−1 and 0.1577 cm−1 respectively, which are in good agreement with the experimental data 2.295 Å, 378.11 cm−1, and 0.1592 cm−1. For the A1Π state of AlBr, the percentage error in Te, Re, and we are 1.50%, 0.13%, and 6.22% compared with the experimental data,[22] and for the a3Π states of AlBr, the percentage error in Te, we, and weχe are 1.98%, 0.20%, and 0.29%.
Overall, our computed spectroscopic constants agree well with the experimental data as well as other available theoretical results. We expect that our methods are accurate for the two molecules.
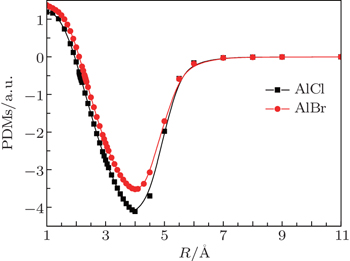
Figures
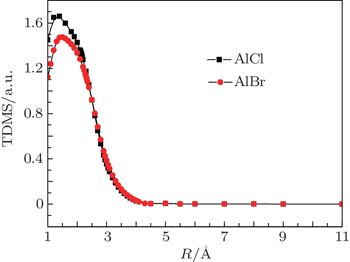
The relationship between the TDMs of the A1Π –X1Σ+ transitions and the internuclear distance is depicted in Fig.
Rapid laser cooling requires sufficiently short lifetimes, which can provide a significant rate (105 s−1–108 s−1) of optical cycling. The radiative lifetimes and radiative width for the transitions from the A1Π state to the ground state X1Σ+ are collected in Table
| Table 2. Estimated radiative lifetimes (in unit ns) and radiative width (in unit cm−1) (in italics) for A1Π → X1Σ+ transitions. Theoretical values obtained by Langhoff et al. in brackets. . |
The radiative lifetimes of the A1Π (ν′) vibrational states are computed to be 5.04 ns–5.76 ns and 8.53 ns–10.83 ns for the first five vibrational levels (ν′ = 0–4) of AlCl and AlBr. So these lifetimes are sufficiently short that AlCl and AlBr meet the criteria as suitable laser cooling candidates. Taking the ν′ = 0 level as an example, the line strengths (Einstein A coefficients) of the A1Π (ν′ = 0) vibrational states for AlCl and AlBr are 1.98×108 s−1 and 1.17×108 s−1, giving lifetimes of 5.04 ns and 8.53 ns (about 1/5 and 2/5 that of the transition in SrF). The radiative widths of the a3Π and A1Π vibrational states are predicted in Table
The calculated FCFs in AlCl for the transition A1Π → X1Σ+ are tabulated in Table
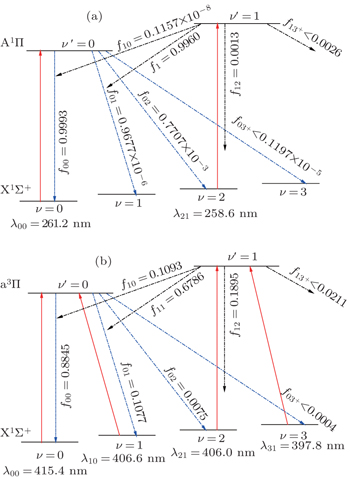 | Fig. 5. Proposed laser cooling schemes for AlCl using the (a) A1Π (ν′) ← X1Σ+ (ν) and (b) a3Π (ν′) ← X1Σ+ (ν) transitions with the calculated FCFs fν′ν and wavelength λν′ν. |
| Table 3. The calculated FCFs fν′ν of AlCl for A1Π (ν′) → X1Σ+ (ν) and a3Π (ν′) → X1Σ+ (ν) transitions. Characteristic base 10 given parenthetically. . |
Table
Turning to AlBr, the FCFs follow the same trend as that of AlCl (see Table
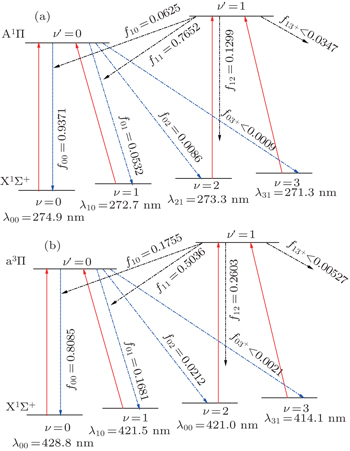 | Fig. 6. Proposed laser cooling schemes for AlBr using the (a) A1Π (ν′) ← X1Σ+ (ν) and (b) a3Π (ν′)← X1Σ+ (ν) transitions with the calculated FCFs fν′ν and wavelength λν′ν. |
| Table 4. The calculated FCFs fν′ν and wavelength λν′ν of AlBr for A1Π (ν′) → X1Σ+ (ν) and a3Π (ν′) → X1Σ+ (ν) transitions. Characteristic base 10 given parenthetically. . |
On the whole, we have identified AlCl and AlBr as promising laser cooling candidates. Compared with AlBr (f00 = 0.9371), AlCl (f00 = 0.9993) is more promising. Because of the hyperfine structure present in Al diatomics, sub- Doppler sisyphus cooling can take place in AlCl and AlBr just like alkali atoms. For AlCl and AlBr, the A1Π ← X1Σ+ transition may be followed by the weak a3Π ← X1Σ+ transition. The recoil temperature (Trecoil = (h/λ)2/2mkB) is the temperature limitation. AlCl and AlBr have greater mass and cooling wavelengths of lower energy (AlCl: 415.4 nm; AlBr: 428.8 nm) than AlF investigated by Wells and Lane.[9] So the recoil temperature for AlCl and AlBr will be smaller than AlF.
We have calculated the X1Σ+, A1Π, and a3Π states of AlCl and AlBr in order to explore the possibility of laser cooling these molecules. First, we investigated the PECs and spectroscopic constants (Re, De, we, weχe, Be, Te). The calculated spectroscopic constants are in good agreement with previous theoretical and experimental data. Second, PDMs and TDMs have been calculated for the X1Σ+ and A1Π states. Finally, we obtained FCFs, radiative lifetimes and radiative widths. The results demonstrate that both molecules have highly diagonally distributed FCFs and short lifetimes. It means that AlCl and AlBr meet the criteria as promising candidates for direct laser cooling. Additionally, we have briefly designed laser cooling schemes for AlCl and AlBr. Since the a3Π → X1Σ+ transition is also strongly diagonal and the lifetimes of the excited a3Π state are relatively long, the A1Π ← X1Σ+ transition may be followed by the weak a3Π ← X1Σ+ transition to obtain a lower Doppler temperature.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 | |
| 39 |