† Corresponding author. E-mail:
The structural, electronic, mechanical, and thermal properties of Pt, Pd, Rh, Ir, Os metals and their alloys PtPdX (X = Ir, Os and Rh) are studied systematically using ab initio density functional theory. The groundstate properties such as lattice constant and bulk modulus are calculated to find the equilibrium atomic position for stable alloys. The electronic band structure and density of states are calculated to study the electronic behavior of metals on making their alloys. The electronic properties substantiate the metallic behavior for all studied materials. The firstprinciples density functional perturbation theory as implemented in quasi-harmonic approximation is used for the calculations of thermal properties. We have calculated the thermal properties such as the Debye temperature, vibrational energy, entropy and constant-volume specific heat. The calculated properties are compared with the previously reported experimental and theoretical data for metals and are found to be in good agreement. Calculated results for alloys could not be compared because there is no data available in the literature with such alloy composition.
The platinum group metals (PGMs) are promising materials for numerous technological applications. The four PGMs Pt, Pd, Ir, and Rh have face-centered cubic (fcc) and other two OS and Ru has hexagonal close-packed (hcp) structure. PGMs are considered as primary ingredients in the automotive, chemical, and petroleum industries. Their thermal stability at high temperatures and the highest shear modulus at room temperature make them superior materials.[1–3] They have been investigated for carbon material protective coatings,[4] heavy-metal-ion sensors,[5] and Re-rocket thrust[6] applications. They are also broadly used in organic chemistry for hydrogenation, hydro-formylation, hydro-boration, hydro-silylation, cyclo-additions,[7,8] and oxidation reactions for the catalytic converters in automobiles. Their incredible catalysis activity increases research interests in these rare elements,[9] but odd with their higher cost. The importance and higher cost of PGMs motivate the researchers for the development of alloys to reduce the material cost and changing in properties to the requirement. The transition metals and their inter-metallic alloys are promising structural materials because of their high melting points, high strength, and good oxidation resistances.[10] During the recent years, the inter-metallic alloys of these PGMs have attracted considerable experimental and theoretical interests of many researchers.[11] Many of the possible alloy compositions have not yet been studied and there are still sizable gaps in the knowledge of the basic properties, especially thermal properties of PGMs and their alloys. There is a considerable difficulty in calculating thermodynamic properties experimentally because they often require high temperatures or pressures and very long equilibration processes.
Ab initio technique based on density-functional theory (DFT)[12–14] has been used to calculate the properties of materials at zero temperature in the last few decades. However, the first-principles approaches in the study of thermal properties are still challenging for researchers. A simplified approach for thermal properties calculation is Debye–Gruneisen theory[15] that maybe used properties calculations[16,17] as well as for compounds.[18] The Car–Parrinello first-principles molecular dynamics simulations method is being used for more accurate thermodynamic properties calculations.[19] However, this simulation method can be a heavy computational task and results may not be realistic enough. Now it is possible to calculate vibrational frequencies at every point of the Brillouin zone with the achievements of density-functional perturbation theory (DFPT).[20,21] This method also has reduced computational time for thermal properties and many applications have been made within DFPT.[22] In this approximation, an harmonic effect is included through the explicit volume dependence of the vibrational frequency. Several thermal expansion calculations for cubic metals based on DFPT have been carried out[23–26] and they give good results about isotropic thermal expansion and related thermal properties. In this paper we apply quasi harmonic approximation with DFPT to the study of the thermal properties of platinum group metals and their alloys. A relatively dense mesh of k points is needed to achieve convergence of phonon frequencies for both metals because of complexities of the Fermi surface.[27]
The first-principles investigation was performed using a pseudo-potential plane wave (PP-PW) method as implemented in QUANTUMES ESPRESSO.[28] We have used super cell with 2 × 2 × 2 dimension to study PGMs and their alloys. Super cell with Pt0.25Pd0.25X0.50 concentration was used for PtPdX (X = Ir, Os and Rh) alloys. Vanderbilt ultra-soft pseudo-potential parameterized by Perdew and Zunger[29,30] was used for calculations. We used ultra soft Vanderbilt formalism with local density approximation (LDA)[31] for the exchange correlation energy of the electrons. The special k-points mesh was integrated to sampled Brillouin zone using the Monkhorst–Pack method.[32] A special k-points mesh of 14 × 14 ×14 was used to produce irreducible Brillouin zone. The pseudo-wave functions were expanded in a plane wave basis set using cut-off energy of 30 Ryd for all concentrations. The chosen cut-off energy and k-point mesh ensure convergence with an accuracy of 10−6 Ryd. DFPT and quasi-harmonic approximation (QHA) code developed by Baroni et al.[28] was used to investigate phonon density of states and thermal properties from optimized structure. The dynamical matrices at arbitrary wave vectors were obtained using Fourier transformation based interpolations for thermal properties calculations. A 10 × 10 × 10 q point mesh was implemented to obtain force constants dynamical matrices in the irreducible Brillouin zone at Γ point. The linear response approach method was used to obtain phonon density of states curves within the frame-work of DFPT[28] as implemented in quantum ESPRESSO.
The parameters such as cell dimension, energy cut-offs, and the k-points used in the study were obtained from their respective convergence tests. These convergence parameters provide a fast way toward the optimized structure.
We have calculated the ground state properties of Pt, Pd, Rh, Ir, Os, and PtPdX (X = Ir, Os, and Rh) to find out the crystal structure. Total energy per unit cell was computed at various lattice parameters to find the equilibrium lattice constant. The total energy minimization method was used to obtain equilibrium lattice constant. For this purpose, Murnaghan’s equation of state was used to evaluate the optimized lattice constants and bulk modulii.[33] The structural parameters, i.e. lattice constants and bulk modulus, were calculated for pure Pt, Pd, Rh, Ir, Os, and their alloys, and are reported in Table
| Table 1. Comparison of calculated lattice parameters and bulk modulus of Pt, Pd, Rh, Ir, Os, and their alloys PtPdX (X = Ir, Os, and Rh) with experimental and other theoretical results. . |
 | Fig. 1. Structural optimization plots for pure metals: (a) Pt, (b) Pd, (c) Ir, (d) Rh, and (e) for Os. |
The electronic properties provide information about the material’s electronic configuration, particularly the existence of prohibited regions of energy and the magnitude in their electronic excitation spectral. Hence, the band structures for Pt, Pd, Rh, Ir, Os, and their alloys PtPdX (X = Ir, Os, and Rh) along various high-symmetry directions were calculated at equilibrium lattice constants. The electronic band structures for pure metals (Pt, Pd, Rh, Ir, Os) are shown in Figs.
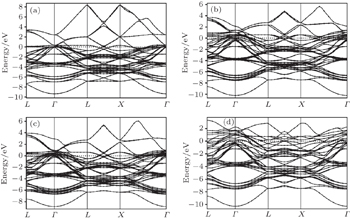 | Fig. 4. Calculated band structures for (a) Pt0.50Pd0.50, (b) Pt0.25Pd0.25Ir0.50, (c) Pt0.25Pd0.25Rh0.50, and (d) Pt0.25Pd0.25Os0.50 alloys. |
The main hybridization was remained between d bands of metals to form their alloys. It was observed from the analysis of band structure of all given metals and their alloys that they all have similar behavior. However, it is also observed that they mainly differ from each other by the energy level relative to the Fermi level. The calculated electronic band structure is in good agreement with previous results for pure metals, i.e. Ir, Rh, Pt, Pd, and Os.[52–56] Calculations of their studied alloys may be reported for the first time. Therefore, there is no previous data available for the comparison to these alloys.
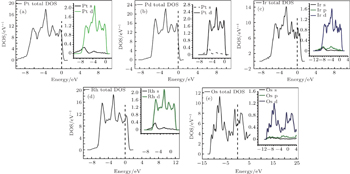
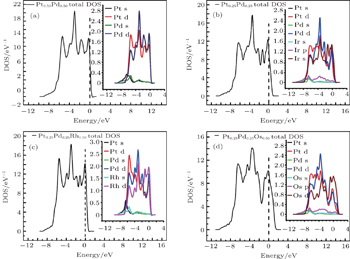
Many physical properties can be studied with the help of density of states (DOS). Specially, it plays a significant role to study the electronic properties of materials and is more important to explore the electronic band structure. From Figs.
In Fig.
In pure metals, i.e., Pt, Pd, and Rh, the conductivity was mostly dominated by s and d hybridization. However, for Ir and Os, hybridization was due to s, p, and d states. The DOS is compared with the results available in literature for Ir, Rh, Pt, Pd, and Os metals.[57–61]
When these metals were mixed to make alloys, the strong hybridization between the d states with small contribution of s states dominated the conductivity. The calculated DOS for various metals and their alloys showed that Rh has high DOS energy at Fermi level among the studied systems. Therefore, it is affirmed that the Rh metal has good conductivity due to its high DOS at Fermi level and its concentration in alloy PtPdRh increases the electronic conductivity.
Materials’ thermal properties have interesting characteristics below the melting temperature. The knowledge of materials’ thermal properties is very important for developments and manufacturing electronic devices.[62,63] Phonon DOS and quasiharmonic approximation were used to obtain thermal properties. Debye temperature, Helmholtz free energy ΔF, internal energy ΔE, entropy S, and constant-volume specific heat Cv at zero pressure are calculated using the following relations:[64]



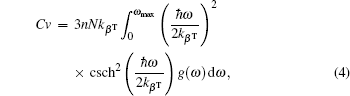
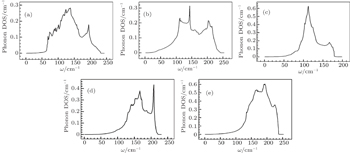
Phonon DOS has strong impact on thermodynamic properties and electron excitation can be investigated with the help of phonon DOS.[65] We have studied total and partial phonon density of states of metals Pt, Pd, Rh, Ir, Os, and their alloys.
Figures
 | Fig. 8. Phonon DOS for (a) Pt0.50Pd0.50, (b) Pt0.25Pd0.25Ir0.50, (c) Pt0.25Pd0.25Rh0.50, and (d) Pt0.25Pd0.25Os0.50 alloys. |
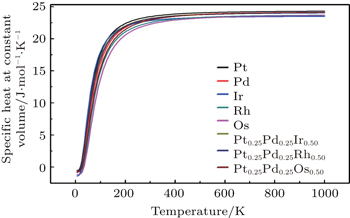
To investigate the more thermal properties, we have calculated the thermal properties such as Debye temperatures, vibration energy, entropy and constant-volume specific heat and are plotted in Figs.
Our calculated results for thermal properties for pure metals, i.e., Ir, Rh, Pt, Pd, and Os, are in good agreement with the previous findings.[74–78] There were no experimental or theoretical results available in the literature for their alloys. Therefore, the results reported in this work will be predictive for future.
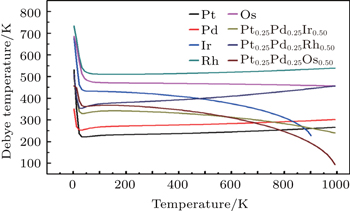 | Fig. 9. Debye temperature variation with temperature for Pt, Pd, Rh, Ir, Os, and PtPdX (X = Ir, Os and Rh) alloys. |
 | Fig. 10. Vibration energy variation with temperature for Pt, Pd, Rh, Ir, Os, and PtPdX (X = Ir, Os, and Rh) alloys. |
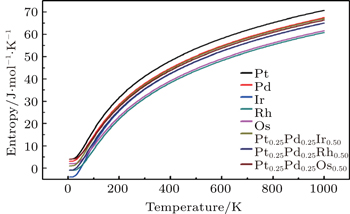 | Fig. 11. Entropy variation with temperature for Pt, Pd, Rh, Ir, Os, and PtPdX (X = Ir, Os, and Rh) alloys. |
We employed the first-principles plane wave pseudo-potential DFT method to analyze the structural, electronic, mechanical, and thermal properties of the Pt, Pd, Rh, Ir, Os, and PtPdX (X = Ir, Os, and Rh) alloys. A super cell with 2 × 2 × 2 dimension was used for all calculations. A super cell with Pt0.25Pd0.25X0.50 concentration was used for PtPdX (X = Ir, Os, and Rh) alloys. The following conclusions are drawn.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 | |
| 39 | |
| 40 | |
| 41 | |
| 42 | |
| 43 | |
| 44 | |
| 45 | |
| 46 | |
| 47 | |
| 48 | |
| 49 | |
| 50 | |
| 51 | |
| 52 | |
| 53 | |
| 54 | |
| 55 | |
| 56 | |
| 57 | |
| 58 | |
| 59 | |
| 60 | |
| 61 | |
| 62 | |
| 63 | |
| 64 | |
| 65 | |
| 66 | |
| 67 | |
| 68 | |
| 69 | |
| 70 | |
| 71 | |
| 72 | |
| 73 | |
| 74 | |
| 75 | |
| 76 | |
| 77 | |
| 78 |