† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant No. 21401064), the Science & Technology Development Program of Henan Province, China (Grant No. 142300410282), and the Program of Henan Educational Committee, China (Grant No. 13B140986).
The thermal stability of Ti@Al core/shell nanoparticles with different sizes and components during continuous heating and cooling processes is examined by a molecular dynamics simulation with embedded atom method. The thermodynamic properties and structure evolution during continuous heating and cooling processes are investigated through the characterization of the potential energy, specific heat distribution, and radial distribution function (RDF). Our study shows that, for fixed Ti core size, the melting temperature decreases with Al shell thickness, while the crystallizing temperature and glass formation temperature increase with Al shell thickness. Diverse melting mechanisms have been discovered for different Ti core sized with fixed Al shell thickness nanoparticles. The melting temperature increases with the Ti core radius. The trend agrees well with the theoretical phase diagram of bimetallic nanoparticles. In addition, the glass phase formation of Al–Ti nanoparticles for the fast cooling rate of 12 K/ps, and the crystal phase formation for the low cooling rate of 0.15 K/ps. The icosahedron structure is formed in the frozen 4366 Al–Ti atoms for the low cooling rate.
The titaninum–aluminide intermetallic compounds have high melting points, high mechanical strength, oxidation resistance, and low density.[1,2] Therefore, these materials have received intensive interest recently in the automotive, aerospace, and power generation industries. A number of experimental[3–13] and theoretical[14–20] studies on Al–Ti systems have been carried out over the past several years. Most of the work is devoted to synthesis,[3–7] microstructure,[8–12] mechanical properties,[9–11] thermophysical properties,[13] etc. Up to now, increasing amount of interest has been paid to the nanostructured TiAl alloys because it can significantly improve the ductility and toughness.[21–23] The thermal and mechanical properties of nanoparticles (NPs) can display some unique behavior associated with the size-dependent properties, such as melting point depression and other low-dimension effects. Theoretically, these studies[14–20] have focused mainly on the rapid solidification,[14,15] synthesis,[16] effect of impurity,[17,18] and alloying reaction.[19,20] However, the understanding of the melting process of nanoparticles has not been addressed satisfactorily because thorough theoretical study on the melting behavior of the Ti core-Al shell at the atomistic level has rarely been reported.
Understanding the thermal stability of bimetallic nanoparticles is particularly important in view of their application. However, experimental investigations of melting are difficult, due to the constraints in conducting accurate experiments at nanoscale. Therefore, molecular dynamics (MD) simulation plays an essential role to study the melting and solidification behaviors. Most investigations have been used successfully in predicting the melting temperature and other properties of bimetallic NPs.[24–28] In our previous works,[29] the heating, cooling, and reactive behavior of core-shell structured Cu–Al NPs was investigated using molecular dynamics simulation. However, there is only one group[19,20] that studied the heating, cooling, and alloying reaction of the Ti-coated Al NPs. Little attention has been paid to the particle size and composition effect in the melting and freezing behaviors of Al–Ti NPs, which is very significant for producing various physical and chemical properties of bimetallic nanoparticles.
Obviously, thermodynamic properties are very important in research and applications, such as melting of the core shell NPs. In this study, we focus on the thermal stability of Al–Ti core-shell NPs with different sizes during continuous heating and cooling processes. Using embedded atom method (EAM), we perform MD simulations on bimetallic Al–Ti NPs with five different sizes, including fixed Ti core radius (Rc = 2 nm), different Al shell thickness (0.3, 0.5, and 0.7 nm), and fixed Al shell thickness (δs = 0.5 nm), different Ti core radius (3 and 4 nm). In all cases, the thermodynamic properties and structure evolution during continuous heating and under different solidification rates will be investigated through the characterization of the total potential energy distribution and radial distribution function (RDF), and some unique behaviors will be revealed.
Ti-core/Al-shell (denoted as Ti@Al) NPs are constructed from an fcc core of Al and an hcp shell of Ti (α-Ti). The bulk lattice constant of Al is aAl = 4.05 Å, while the bulk lattice constants of Ti are aTi = 2.95 Å and cTi = 4.68 Å. In order to trace the influence of the core/shell ratio, we have constructed Ti@Al NPs with a fixed core radius and different shell thicknesses, as well as a fixed shell thickness and different core radii for comparison in this study. For the fixed Ti core radii (Rc = 2 nm, contains 1904 Ti atoms), the thicknesses of Al shell can take values of 0.7, 0.5, or 0.3 nm (3662, 2462, or 1280 Al atoms, denoted as NP1, NP2, or NP3, respectively). Similarly, for the fixed Al shell thickness (δs = 0.5 nm, contain 4710 and 7866 Al atoms, respectively), the radius of Ti core is measured to be 3 and 4 nm (6493 and 15176 Ti atoms, denoted as NP4 and NP5, respectively). Ti@Al NPs is separated by a gap of 3.0 Å between the core and shell atoms, as illustrated schematically in Fig.
All molecular dynamics (MD) simulations are carried out with the LAMMPS software package.[30] The interatomic interactions are described by an embedded atom method (EAM) potential developed by Zope and Mishin.[31] The EAM potential is constructed by fitting to a large experimental database as well as ab initio data. This potential has been successfully used in previous investigations of fcc random alloys and NPs, demonstrating an accurate description of elastic properties and alloying reaction.[19,32] Therefore, the present EAM potential for investigating the Ti–Al system is reliable and accurate. An energy minimization process is performed for all the NPs before the MD simulation started. The time step in all calculations is 1.0 fs, which leads to quite stable dynamics trajectories for the system. Non-periodic boundary conditions are used to ensure the simulation of isolated NPs during the simulation process. All the MD simulations are carried out in the canonical ensemble (NVT) with the application of the Nosé–Hoover thermostat. For the heating simulation, each nanoparticle is first relaxed for 100 ps at 300 K. Then a constant rate of heating (0.6 K/ps) is employed in the heating simulation; i.e., NP1, NP2, and NP3 are heated gradually for 2×106 time steps, which is equivalent to 2000 ps, with the temperature rising from 300 K to 1500 K, while NP4 and NP5 are heated stepwise from 300 K to 2100 K with a time step of 3000 ps. For the solidification simulation, an additional relaxation time of 100 ps at 1500 K is conducted before implementing the cooling process. Three quenching rates for solidification are simulated: 12 K/ps, 6 K/ps, and 0.15 K/ps, corresponding to runtimes of 1.0 × 105, 2.0 × 105, and 8.0 × 106 fs.
The thermodynamics properties, the characteristics, and the progress of the Ti@Al NPs melting during the heating process can be obtained from data records by MD simulations. Generally, the transition temperature from the solid to liquid phase is usually identified by investigating the variation in the thermodynamic properties such as potential energy and specific heat. Note that the specific heat can be induced as a function of temperature according to the following equation:[33]

For a constant heating rate of 0.6 K/ps, the temperature dependence of potential energies and specific heat for five Ti@Al NPs is shown in Fig.
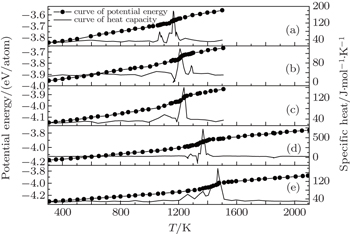 | Fig. 2. Temperature-dependent potential energies and specific heat for five different NPs during the heating process. (a) NP1; (b) NP2; (c) NP3; (d) NP4; (e) NP5. |
| Table 1. Melting temperature Tm for different NPs. . |
In order to visualize the melting process and to shed light on the melting mechanism, we have investigated atomistic structural evolution of the Ti@Al NPs during the heating process. As a representative, NP2 has been used to exhibit the thermal evolution of core/shell NPs under the continuous heating process. Figure
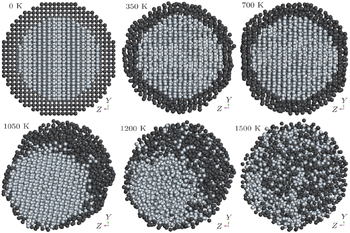 | Fig. 3. Snapshots of the evolution of NP2 during a heating process (Al atoms are colored in dark grey, while Ti atoms are in grey). |
Radial distribution function (RDF) is of importance to the analysis of particle structures and properties. RDF gives the local atomic arrangement and is a tool to distinguish between solids and liquids. The heights of the RDF peaks manifest the local order of structure. While crystalline solids are characterized by a repeating sequence of sharp peaks separated by distances between neighbors, RDF for liquids has few peaks at short distances and no long range order. Here, we use RDF to support above conclusion that the shells melt first and then the cores for core/shell Ti@Al NPs. We calculate the RDF of the core and the shell for Ti@Al core/shell NP2 of 4366 atoms, the results shown in Fig.
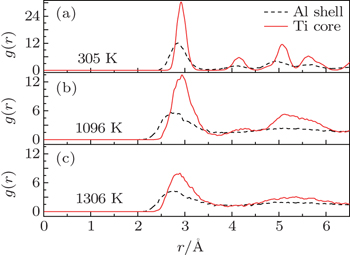 | Fig. 4. RDF of core and shell for Ti@Al core/shell NPs of 4366 atoms (NP2) at 305, 1096, and 1306 K. |
Single-particle MD simulations are also conducted on pure Ti NPs to establish a comparative basis for the heating study of the five different NPs. The melting temperature for three different-sized pure Ti NPs is also listed in Table
The solidification simulation continues after heating the five different Ti@Al NPs. As a representative, NP2 has also been used to exhibit the thermal evolution of Ti@Al NPs under the continuous cooling process.
Reference [35] reported that there are some difficulties in the amorphization process of TiAl alloys using the conventional rapid quenching technique. To validate this conclusion, first of all we calculate the potential energies for three cooling rates of NP2, with the results shown in Fig.
| Table 2. Crystallizing temperature Tc, and glass formation temperature Tg for different NPs. . |
Here, we calculate RDF to monitor particle structure evolutions during the simulation, as shown in Fig.
 | Fig. 6. RDF of NP2 under different cooling rates: (a) cooling rate of 12 K/ps and (b) cooling rate of 0.15 K/ps. |
In order to provide intuitive structure of the final quench product under different cooling rates, we present the final snapshot of the quenching as shown in Fig.
We have employed molecular dynamic simulations to investigate the thermal stabilities of Ti@Al NPs with different sizes and components under continuous heating and cooling. The analyses of potential energy and specific heat during the heating simulation have revealed that the effect of component on the melting temperature can be found. We have found that the liquid shell and solid core coexist for core/shell Ti@Al NPs during heating process, which would raise an interest for the catalytic properties of bimetallic core/shell NPs. The cooling rate significantly affects the final phase formation. A glass phase is formed for a fast cooling rate of 12 K/ps, while a crystal structure is formed for a slow cooling rate of 0.15 K/ps. The icosahedron structural is formed in the frozen 4366 Al–Ti atoms for the low cooling rate. The glass formation temperature and the crystallizing temperature can increase with the size of core/shell Ti@Al NPs.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 |





