† Corresponding author. E-mail:
Project supported by the National High Technology and Development Key Program, China (Grant No. 2015AA034201), the National Natural Science Foundation of China (Grant Nos. 11234013 and 11264014), the Natural Science Foundation of Jiangxi Province, China (Grant Nos. 20133ACB21010, 20142BAB212002, and 20132BAB212005), and the Foundation of Jiangxi Provincial Education Committee, China (Grant Nos. GJJ14254 and KJLD14024).
From first principle calculations, we demonstrate that LiXS2 (X = Ga, In) compounds have potential applications as cathode materials for Li ion batteries. It is shown that Li can be extracted from the LiXS2 lattice with relatively small volume change and the XS4 tetrahedron structure framework remains stable upon delithiation. The theoretical capacity and average intercalation potential of the LiGaS2 (LiInS2) cathode are 190.4 (144.2) mAh/g and 3.50 V (3.53 V). The electronic structures of the LiXS2 are insulating with band gaps of 2.88 eV and 1.99 eV for X = Ga and In, respectively. However, Li vacancies, which are formed through delithiation, change the electronic structure substantially from insulating to metallic structure, indicating that the electrical conductivities of the LiXS2 compounds should be good during cycling. Li ion migration energy barriers are also calculated, and the results show that Li ion diffusions in the LiXS2 compounds can be as good as those in the currently widely used electrode materials.
It is now universally recognized that lithium ion batteries (LIBs), with advantages like high energy density and long cycling life, are one of the most important energy storage sources and being widely used in various consumer electronics industries. The most promising application of the LIBs in the near future is in the power supplies for electrical vehicles (EVs).[1–3] However, the comprehensive performance of the currently commercialized LIBs is difficult to fully satisfy the market demands of the EV industry in terms of the energy density, cycling life and safety. Many efforts, either experimentally or theoretically, have been made to improve the comprehensive performance of the LIBs.[4–7] As for the safety problem, the inflammable organic liquid electrolyte is (at least partly) responsible for most of the fire or explosion accidents occurring in either the consumer electronic and power LIBs. One alternative is to develop the all solid state LIBs, in which non-inflammable solid state electrolytes are used to replace the liquid electrolyte and therefore the safety of the battery system is enhanced.[8] In addition to the better safety, Li metal can be directly used as anode for all solid state LIBs, which is beneficial to enhancing the energy density of the battery system. On the other hand, Li metal anode allows us to select the cathode with more flexibilities, because Li anode can be used as the Li source and thus the cathode material does not necessarily contain Li element. Furthermore, all-solid-state Li battery has a wider electrochemical window and the solid/solid interface is electrochemically more stable than the solid/liquid interface. These features are beneficial to obtaining full utility of the cathode’s capacity.[9,10]
In order to accelerate the development of all-solid-state LIBs, it is important to discover or design novel solid state electrolyte materials that possess both good electrochemical stability and high Li ion conductivity. There are three types of solid state electrolyte materials that are reported in the literature. The most widely studied type is the inorganic crystalline solid state electrolyte, which includes the perovskite structured materials,[11] the garnet structured materials,[12,13] the NASICON,[14] and LISICON[15] structured materials, etc. Generally speaking, these inorganic solid state electrolytes are electrochemically very stable with high electrochemical window while the Li ion conductivity is not good enough to satisfy the industrial application, particularly the Li diffusion across the solid electrode/electrolyte interface leads to large interfacial impedance. Polymer electrolyte (PEO) is another type of solid state electrolyte and has been widely studied in the literature in the past years.[16,17] Unlike the inorganic solid state electrolyte, the plasticity of PEO is very good and therefore the electrode/electrolyte contact is good with PEO electrolyte. However, the Li ionic conductivity in PEO ranges from 108 S/cm to 10−4 S/cm at temperatures between 40 °C and 100 °C,[18] and thus the allsolidstate LIBs with using PEO as electrolyte must be operated under high temperatures.[19] The third type of solid state electrolyte is non-crystalline glassy sulfide material that contains Li2S and other sulfide as the main component. The non-crystalline sulfide electrolyte has higher Li ion conductivity than the crystalline oxide electrolyte in general, while this type of material is very reactive to H2O molecule in air and therefore becomes very unstable in air.[20,21]
Recently, Kamaya et al.[22] reported a novel crystalline solid state electrolyte Li10GeP2S12 (LGPS), with an electrochemical window width of 5 V and Li ion conductivity reaches 1.2 × 10−2 S/cm under room temperature. This value is the highest in Li ion conductivities ever reported so far in the literature for solid state electrolytes. As a promising solid state electrolyte, the lithium ion diffusion mechanism,[23,24] the phase and structural stability,[25] the mechanical properties,[26] and the electrochemical performances of LGPS[27] have been studied recently.
Although the LGPS has very good Li ionic conductivity, there is still a long way to go before it can be widely used in the all-solid-state LIBs. The interfacial problem at the electrode and the electrolyte boundary occurs for all kinds of sulfide solid state electrolytes.[28] The electrode/electrolyte interface in an Li/LGPS/Li half battery reduces the Li ion conductivity by more than one order of magnitude, indicated from the ion conductivity measurement of the bulk material.[27] More importantly, from density functional theory calculations, it is shown that the LGPS is electrochemically unstable at the electrode/electrolyte interface.[29] The interfacial reaction decomposes the LGPS in the interface area, and therefore the electrochemical performance of the battery becomes deteriorated. Technically, a thin buffer layer (several nanometers thick) between the oxide cathode and the sulfide solid state electrolyte can reduce the interfacial resistance and hence the rate capacity can be increased. Those buffer layers include LiNbO3[30,31] and Li2O-SiO2 glasses,[32] and Li4Ti5O12,[33] while the oxide cathodes include LiCoO2 and LiMn2O4.[34] The improved electrochemical performance via introducing the thin buffer layer is attributed to the lowering of the space-charge potential in the interface area.
As discussed above, there are two major problems concerning the electrode/electrolyte interface, which are recognized in the literature, namely, the reaction of sulfide solid electrolyte with the electrode, and the formation of space-charge layer in the interface region. These problems block the way of rapidly developing the all-solid-state LIB with using sulfide electrolytes. Although coating can lower the space-charge potential and prevent the reaction between the sulfide electrolyte and the cathode, it makes the manufacture procedure more complicated and the cost increased. Another strategy is to find novel cathode materials that are more compatible with the sulfide electrolyte. If we can use Li sulfide compound cathode rather than oxide cathode, the interfacial reaction and the space-charge induced Schottky barrier can be substantially reduced.
With the development of the computer simulation techniques, it is possible to screen novel materials using high-throughput computational technology. Materials Genome Initiative (MGI) combines the high-throughput computational materials sciences with available experimental data to accelerate the development of novel materials. Using the main idea of the MGI, Hautier et al. identified two novel cathode materials with capacity values higher than 200 mAh/g.[35] In the present paper, from high-throughput first principle calculations, we search for all possible Li sulfide compounds. Selection criteria include those for the theoretical capacity, the intercalation voltage, the electronic and Li ion conductivity, and the structural stability.[36] We identify two Li sulfide compounds that have potential application as cathode material for all-solid-state LIB with sulfide electrolyte.
In order to use the MGI idea to fast screen the physical and chemical properties of the Li compounds using the high-throughput computational techniques, we develop a computational platform that supports the first principle calculation and the output result analysis spontaneously. The architecture of the platform and the details on the data collection and data mining are introduced in Ref. [37]. The screening procedure and criteria for Li ion battery materials are introduced in Ref. [36]. In the present work, we select all Li sulfide compounds as candidate from the inorganic crystal structure database (ICSD). The theoretical Li storage capacity and the average intercalation potential criterion are larger than 140 mAh/g and higher than 3.0 V. Then the structural stability is evaluated through analyzing the structural change and calculating the volume change upon Li extraction and insertion. If these criteria are satisfied, the Li ion migration energy barriers and the electronic structures are calculated to evaluate the Li ion diffusion and electrical conduction. Finally, we identify that two compounds, namely the LiGaS2 and LiInS2 satisfy all criteria and have potential applications as cathode materials for allsolidstate LIBs.
First principle calculations are performed using the Vienna ab initio simulation package (VASP) within the projector augmented-wave (PAW) approach.[38] The ground state of the electronic structure is described with density functional theory (DFT) using the generalized gradient approximation (GGA) with PW91 exchange correlation functional.[39] The wave functions are expanded into plane waves and the cutoff energy is selected to be 520 eV. The calculation of density of states (DOS) is smeared by the Gaussian smearing method with a smearing width of 0.02 eV. The Li migration pathway and energy barrier are optimized by the nudged elastic band (NEB) method.[40]
The average lithium extraction potential is calculated from[41–43]
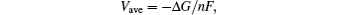
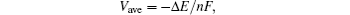
From our first principle calculation, we identify that all Li ions can be delithiated from the LiXS2 lattice and therefore the theoretical Li storage capacities of the two materials are calculated to be 190.4 mAh/g and 144.2 mAh/g for LiGaS2 and LiInS2, respectively. On the other hand, the average intercalation potentials are calculated to be 3.50 V and 3.53 V versus Li/Li+ for the LiGaS2 cathode and LiInS2 cathode, respectively. Therefore, the corresponding energy densities of the two cathode materials are 665.6 Wh/kg and 468.7 Wh/kg, which are comparable to those of the currently widely used oxide cathodes like spinel structured LiMn2O4 material (475 Wh/kg) and the olivine structured LiFePO4 material (586 Wh/kg).
The LiGaS2 and LiInS2 compounds were synthesized several decades ago.[44,45] The crystal structures of compounds LiXS2 (X = Ga, In) both belong to the orthogonal crystal system with space group of Pna21[45,46] as shown in Fig.
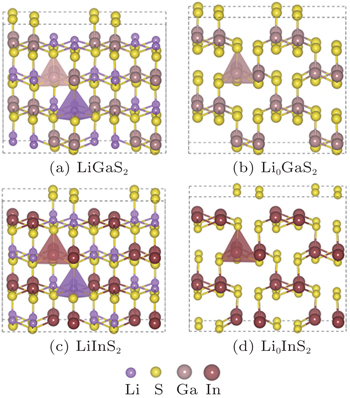 | Fig. 1. Optimized structures of the LiXS2 (X = Ga, In) compounds before ((a), (c)) and after ((b), (d)) delithiation. |
In Table
| Table 1. Theoretical lattice parameters for a 2 × 2 × 2 supercell of the LiXS2 (X = Ga, In). . |
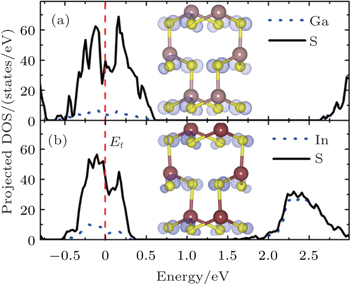
To achieve high charge and discharge speed, the electrical conductivity values of the electrode materials of the Li ion batteries should be high. In the present work, we calculate the electronic structures of the LiXS2 cathodes (including their delithiated states Li0XS2) and the electrical conductivity values can be evaluated qualitatively from the analyses of the electronic structures. The calculated total density of states (TDOS) is given in Fig.
 | Fig. 2. The TDOSs of the LiGaS2 (a) and LiInS2 (b) cathode materials before (solid black lines) and after (dashed blue lines) delithiation. The Fermi level is set to be 0 eV. |
The insulating electronic structure indicates the poor electrical conductivity, which is not good for the rate performance. However, this is a common problem that has already existed for most of the other cathode materials. For example, it is shown that the electronic structures of the LiCoO2 and LiMn2O4 cathodes with band gaps of 2.7 eV[53] and 1.2 eV,[54] respectively are also insulating. Furthermore, the band gap of the olivine structured LiFePO4 cathode is even as high as 3.7 eV.[55]
Technically, defects can change the electronic structures of these insulating materials, thus helping to improve their electrical conductivities. In the present work, we show that Li vacancies, which are created in the delithiation process, can change the electronic structures of the LiXS2 compounds substantially from insulating to metallic structure. As shown in Fig.
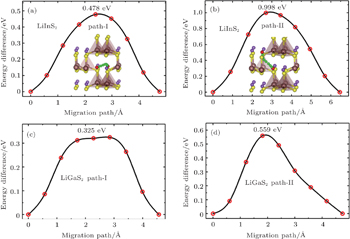
The rate performance of an electrode material is also strongly related to the Li ion diffusion behavior in the bulk material. From the analyses of the Li ion distribution and the symmetry of the crystalline structure, we figured out two independent Li diffusion pathways (path-I and path-II in Fig.
In this study, two compounds LiXS2 (X = Ga, In) are identified as potential cathode materials for Li ion batteries. The first principle calculations show that their basic physical and chemical properties, including the structural stabilities, the electrical and Li ion conductivities, the intercalation potentials, are suitable for use as cathode materials. As the LiXS2 are sulfide, they are particularly suitable for all-solid-state LIBs with using sulfide solid state electrolyte. From the obtained theoretical Li storage capacities and the average intercalation potential, the corresponding energy densities of the two cathode materials are calculated to be 665.6 Wh/kg and 468.7 Wh/kg, which are comparable to those of currently widely used oxide cathodes. Although the electronic structures of the LiXS2 compounds are insulating, their electrical conductivities can be improved substantially upon delithiation, as the electronic structure changes from insulating to metallic structure when Li vacancies are induced. Furthermore, from the calculated Li ion migration energy barriers in the LiXS2 compounds, we identify a three-dimensional Li diffusion network in each of these materials with energy barriers that are comparable to those of the currently widely used electrode materials, indicating that the Li ion migration dynamic performances of the LiXS2 compounds are acceptable for the cathode materials.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 | |
| 39 | |
| 40 | |
| 41 | |
| 42 | |
| 43 | |
| 44 | |
| 45 | |
| 46 | |
| 47 | |
| 48 | |
| 49 | |
| 50 | |
| 51 | |
| 52 | |
| 53 | |
| 54 | |
| 55 | |
| 56 | |
| 57 | |
| 58 | |
| 59 | |
| 60 |