† Corresponding author. E-mail:
‡ Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant No. 51002063) and the International Science and Technology Cooperation Program of Science and Technology Bureau of Changchun City, China (Grant No. 12ZX68).
ZrO2 nanodots are successfully prepared on LaAlO3 (LAO) (100) substrates by photo-assisted metal-organic chemical vapor deposition (MOCVD). It is indicated that the sizes and densities of ZrO2 nanodots are controllable by modulating the growth temperature, oxygen partial pressure, and growth time. Meanwhile, the microwires are observed on the surfaces of substrates. It is found that there is an obvious competitive relationship between the nanodots and the microwires. In a growth temperature range from 500 °C to 660 °C, the microwires turn longest and widest at 600 °C, but in contrast, the nanodots grow into the smallest diameter at 600 °C. This phenomenon could be illustrated by the energy barrier, decomposition rate of Zr(tmhd)4, and mobility of atoms. In addition, growth time or oxygen partial pressure also affects the competitive relationship between the nanodots and the microwires. With increasing oxygen partial pressure from 451 Pa to 752 Pa, the microwires gradually grow larger while the nanodots become smaller. To further achieve the controllable growth, the coarsening effect of ZrO2 is modified by varying the growth time, and the experimental results show that the coarsening effect of microwires is higher than that of nanodots by increasing the growth time to quickly minimize ZrO2 energy density.
ZrO2 with excellent physical and chemical properties is attracting extensive interest in many fields nowadays. Because of the high-refractive index of ZrO2, ZrO2/Epoxy nano-composites are considered to be used as LED encapsulations.[1] ZrO2 nanostructures have already been used as artificial pinning centers (APCs) to strongly pin the quantized vortices in high temperature superconductors (HTS).[2] ZrO2 is the only transition metal oxide which possesses not only acidic sites but also alkaline sites and can be used as both catalyst and catalyst carrier.[3–5] Especially, owing to the excellent chemical stability and larger relative surface area, catalytic performance of ZrO2 nanostructure can be much better than bulk material.[6,7] Based on these charming applications, an investigation about the way to grow controllable nanostructures of ZrO2 is essential.
Varieties of methods have been utilized for ZrO2 fabrication, such as pulsed laser deposition (PLD),[8] atomic layer deposition (ALD),[9] and sputtering.[10] Compared with these methods, metal–organic chemical vapor deposition (MOCVD) technology is a proven fabrication technology and versatile process for high quality/large-area production of materials with high growth rates.[11–15] Various papers have reported the preparation of ZrO2 films by MOCVD technology, and discussed the growth mechanism.[16,17] However, there are no reports on ZrO2 nanostructures prepared by MOCVD.
Compared with the ordinary heat treatment technology, the photo-activation in the photo-assisted process possesses some advantages,[18,19] for example, superconducting YBa2Cu3O7−δ (YBCO), GdBa2Cu3O7−δ (GdBCO) films prepared by photo-assisted MOCVD have good alignments and high growth speeds.[20–22] Experimental results indicated that photo-assisted annealing had obviously a higher oxidization rate for the YBCO films in one oxygen atmosphere than thermal annealing.[23] In the present work, the photo-assisted MOCVD is applied to the preparation of ZrO2 nanostructure for the first time.
Quite a lot of reports show that growth parameters have significant effects on the morphologies and physical properties of nanostructures prepared by MOCVD. For example, in a growth temperature range from 500 °C to 610 °C, the island density of GaSb first increases and then decreases.[24] Furthermore, there is a linear relationship between the size of ZnO nanodots and the growth time.[25] Oxygen partial pressure is also an important factor in the growth of Y2O3 by MOCVD.[26] So the effect of growth parameters on the surface morphologies of ZrO2 is worthy of investigation. Our study shows that the morphologies of ZrO2 could be controlled through systematically modulating the growth parameters, such as growth temperature, oxygen partial pressure and growth time. The effects of the growth parameters on the surface morphologies of ZrO2 are systematically analyzed and the growth mechanism is discussed.
Hetero-epitaxial ZrO2 was grown on a single crystal LAO (100) substrate with a perovskite pseudocubic structure by a photo-assisted MOCVD system. The structure of the photo-assisted MOCVD system has been reported in our previous work.[21] Solid metal–organic source of Zr(tmhd)4 (tmhd = 2, 2, 6, 6-tetramethyl-3, 5-heptanedionate) was used as a precursor of Zr. A mixture containing oxygen and nitrous oxide with a volume ratio of 3:2 was used as an oxidizing agent.[27] Zr(tmhd)4 was sublimated at 230 °C in a carrier gas of high purity (99.999%) argon with a molar flow rate at 2 × 10−4 mol/min to participate in the reaction. The total pressure in the reaction chamber was controlled and set at 1000 Pa. Oxygen partial pressure was determined by the flow rate of the oxidizing gas, with the flow rate of argon kept constant.
For these experiments the dependence of the morphologies of ZrO2 on the growth parameters was investigated. Those growth parameters including growth temperature, oxygen partial pressure, and growth time were set and controlled according to a set of primary experiments. Firstly, the growth temperature was varied from 500 °C to 660 °C, while the oxygen partial pressure and growth time were set at 752 Pa and 60 s, respectively. Secondly, ZrO2 nanostructures were fabricated at various oxygen partial pressures ranging from 451 Pa to 752 Pa. The growth temperature was kept constant (at 600 °C), and the growth time was set to 60 s. Finally, the growth times were set to 20 s, 40 s, and 60 s while the oxygen partial pressure was set to 752 Pa at the growth temperature of 600 °C.
Surface morphologies of ZrO2 were investigated by an atomic force microscope (AFM; iCON, Veeco) with the tapping mode at room temperature. Statistical analyses of AFM images including the diameter, height, and density of nanodots as well as the length, width, and height of microwires were carried out by NanoScope Analysis software.
The effect of growth temperature on the surface morphologies of ZrO2 nanostructures is determined at constant oxygen partial pressure (752 Pa) and growth time (60 s) as shown in Fig.
Figure
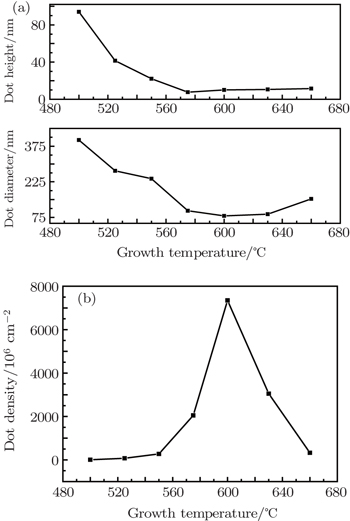 | Fig. 2. (a) Average height and diameter of ZrO2 nanodots, and (b) density of nanodots versus growth temperature. |
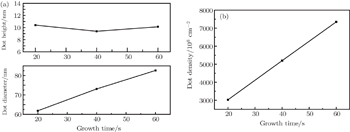
The controllable growth of ZrO2 nanodots is difficult to achieve by an isolated set of experiments. For investigating the kinetic processes involved in the growth of ZrO2 nanodots on LAO (100) substrates, a series of samples is prepared at oxygen partial pressures from 451 Pa to 752 Pa at fixed growth temperature (600 °C) and growth time (60 s) as shown in Fig.
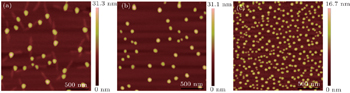 | Fig. 3. The 2 μm × 2 μm two-dimensional AFM images of ZrO2 nanodots on LAO (100) at different oxygen partial pressures: (a) 451 Pa, (b) 602 Pa, (c) 752 Pa. |
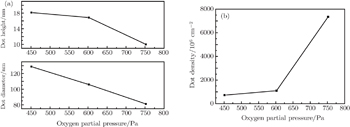 | Fig. 4. (a) Average height and diameter of ZrO2 nanodots, and (b) density versus oxygen partial pressure. |
For further discussing the mechanism of the growth process of nanodots, the growth time is modulated at a fixed temperature of 600 °C and oxygen partial pressure of 752 Pa, the effect of growth time on the ZrO2 nanostructures is as follows (Fig.
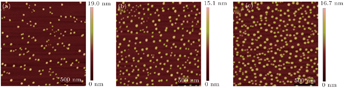 | Fig. 5. The 2 μm × 2 μm two-dimensional AFM images of ZrO2 nanostructures on LAO (100) at various growth times: (a) 20 s, (b) 40 s, (c) 60 s. |
Surprisingly, the anisotropic ZrO2 microwires are found on LAO (100) substrates. These mutually perpendicular microwires constitute a network structure. Owing to the large sizes of microwires compared with those of the nanodots, the nanodots cannot be seen. It is found that growth conditions also have a great influence on the morphologies of microwires. Besides, experimental results indicate that the two different ZrO2 morphologies are in competition.
The surface morphologies of ZrO2 microwires grown at various growth temperatures are shown in Fig.
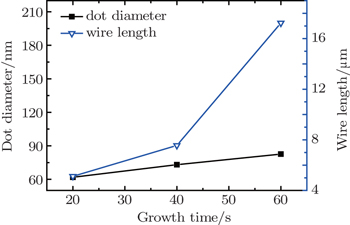
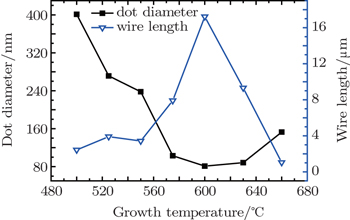 | Fig. 8. Diameter of nanodots and length of microwires versus growth temperature, showing their competitive relationship. |
In order to further understand the competitive relationship between the ZrO2 nanodot and microwire, the microwires are prepared at various oxygen partial pressures, with growth temperature (600 °C) and time (60 s) kept unchanged. Figure
 | Fig. 9. The 50 μm × 50 μm two-dimensional AFM images of ZrO2 microwires on LAO (100) at different oxygen partial pressures: (a) 451 Pa, (b) 602 Pa, (c) 752 Pa. |
 | Fig. 10. Variations of diameter of nanodots and the length of microwires with oxygen partial pressure, showing their competitive relationship. |
In addition, the influence of growth time on the competition between the nanodots and the microwires is also investigated. As mentioned above, the ZrO2 nanodots tend to laterally grow rather than vertically grow. As for the ZrO2 microwires, a similar law is found in Fig.
 | Fig. 11. The 50 μm × 50 μm two-dimensional AFM images of ZrO2 microwires on LAO (100) at various growth times: (a) 20 s, (b) 40 s, (c) 60 s. |
In this work, the growth of ZrO2 nanostructures on LaAlO3 (LAO) (100) substrates by the photo-assisted MOCVD is investigated, and two kinds of completely different surface morphologies of ZrO2 are observed, i.e., ZrO2 nanodots and microwires. The experimental results show that the two kinds of surface morphologies of ZrO2 depend strongly on the growth parameters. At high oxygen partial pressure, the growth of microwires tends to be dominant. With increasing the growth time, the microwires of ZrO2 preferentially coarsen to minimize ZrO2 energy density. Similarly, there is a clear competitive relationship between the ZrO2 nanodot and microwire in the process of modulating the growth temperature. When grown at low temperature, the nanodot presents the shape of large and sparse ones because of the less nucleation sites and the low migration rate of atoms. With increasing the growth temperature, the nanodots become small and dense. When the temperature increases further, a high surface migration rate could result in a significant coarsening. The morphologies of nanodots are characterized by large and sparse ones again. However, the trend of microwires is totally different. Compared with the size at 600 °C, the size of microwires is small at 500 °C, and so is it at 660 °C. Our experimental results demonstrate the feasibility for the controllable growth of ZrO2 by photo-assisted MOCVD.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 |