† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 61274074, 61271070, and 61574100).
Ultrathin VO2 nanobelts with rough alignment features are prepared on the induction layer-coated substrates by an ethylenediaminetetraacetic acid (EDTA)-mediated hydrothermal process. EDTA acts as a chelating reagent and capping agent to facilitate the one-dimensional (1D) preferential growth of ultrathin VO2 nanobelts with high crystallinities and good uniformities. The annealed induction layer and concentration of EDTA are found to play crucial roles in the formation of aligned and ultrathin nanobelts. Variation in EDTA concentration can change the VO2 morphology of ultrathin nanobelts into that of thick nanoplates. Mild annealing of ultrathin VO2 nanobelts at 350 °C in air results in the formation of V2O5 nanobelts with a nearly unchanged ultrathin structure. The nucleation and growth mechanism involved in the formations of nanobelts and nanoplates are proposed. The ethanol gas sensing properties of the V2O5 nanobelt networks-based sensor are investigated in a temperature range from 100 °C to 300 °C over ethanol concentrations ranging from 3 ppm to 500 ppm. The results indicate that the V2O5 nanobelt network sensor exhibits high sensitivity, good reversibility, and fast response-recovery characteristics with an optimal working temperature of 250 °C.
Assembling low-dimensional nanomaterials have attracted increasing interest due to their large specific surface areas and dimensions comparable to the Debye length,[1,2] and have various applications in nanodevices.[3,4] Vanadium oxide nanobelts with a rectangular cross section have attracted wide attention because of their versatile redox-dependent properties and potential applications in lithium batteries,[5] catalysts,[6] gas sensors,[7] and thermochromic devices.[8] As is well known, the size especially the thickness, crystallinity and alignment of nanobelts have great effects on their properties. Thus, the exploration of effective strategies on tailoring the microstructure such as the thickness and geometric arrangement of vanadium oxide nanobelts is desirable.
Chemical routes, such as the surfactant-assisted hydrothermal process are usually used to synthesize low-dimensional nanostructures. Up to now, different vanadium oxide nanostructures, such as V2O5 nanorods, V2O5 nanowires, and VO2 nanobelts, have been reported to be synthesized via the hydrothermal method.[9–11] For the solution-based synthesis, organic modifiers like urea, sodium dodecylsulfate, and chelating agents have been proved to be effective in controlling the final shape of the nano- and micro-crystals, such as nanobelts,[12] sheaf-like nanostructures,[13] and nanorods.[14] They serve as “capping reagent” to break the limitation of the intrinsic growth habit and then govern the crystal growth dynamically. However, the hydrothermally synthesized low-dimensional vanadium oxide nanostructures usually show a morphology of disordering assembly.[12,15–17]
In the present work, with the further consideration of the necessity of ordering arrangement for one-dimensional (1D) nanobelts, we develop a two-step process, i.e., inductive nucleation followed by EDTA-mediated hydrothermal growth, to realize aligned assembly of ultrathin VO2 nanobelts on a substrate. The induction layer performed on the substrate provides initial nucleation sites, and the EDTA serves as chelating and capping agent to modify the growth dynamics of vanadium oxide along different directions. The in-situ formation mechanism of VO2 nanobelts involving the effect of EDTA concentration is proposed. Furthermore, ultrathin V2O5 nanobelts are prepared via a mild annealing of ultrathin VO2 nanobelts at 350 °C in air. To the best of our knowledge, a similar research on EDTA-mediated growth of aligned ultrathin VOx nanobelts has not been reported. The as-synthesized nanobelts with ultrathin structures and certain alignments will have significant applications in nanodevices. Especially, V2O5 is known as a promising gas-sensing material for gas sensor application due to its attractive sensing performances to various gases. Thus, to demonstrate the potential applications of the as-prepared ultrathin vanadium oxide nanobelts, the gas-sensing properties of V2O5 nanobelt networks to ethanol are further evaluated and analyzed.
To prepare VO2 nanobelts, a two-step process was performed. In the first step, an induction layer was prepared on a cleaned alumina substrate by spin-coating 0.05-M NH4VO3 solution with a pH value of 2.1–2.5 followed by thermal annealing at 600 °C in air for 2 h. The spin coating was repeated four times to ensure a uniform distribution and adequate coverage of the induction particles on the substrate after annealing. Prior to spin-coating of the NH4VO3 solution, a pair of interdigitated Pt electrodes each with a thickness of 100 nm was deposited on the cleaned alumina using the magnetic sputtering method. In the following step, the induction particles-coated substrate was submerged in a mixture solution of NH4VO3 and EDTA, which was sealed in a 100-ml Teflon-lined stainless autoclave. The molar ratio of NH4VO3 to EDTA was 6 and the pH value of the solution was about 2.5. Then the hydrothermal reaction proceeded at 180 °C for 12 h. After the autoclave was cooled to room temperature naturally, the alumina substrate coated with VO2 nanobelts was taken out and rinsed, and then dried at 80 °C for 6 h in a vacuum. To further prepare the V2O5 nanobelts, the above VO2 nanobelts were heated at 350 °C in air for 1 h, and then cooled naturally. The samples were characterized with a field emission scanning electron microscope (FESEM, Hitachi S4800), x-ray diffraction (XRD, RIGAKU D/MAX 2500V/PC, Cu Kα radiation), and field emission transmission electron microscopy (FETEM, TECNAI G2F-20).
Gas-sensing properties of V2O5 nanobelt networks were investigated in a home-built static gas-sensing characterization system as shown in our previous work.[18] The sensor was placed on the heating plate and a predetermined amount of ethanol gas was introduced into the chamber directly to reach a desired concentration. The resistance change of the sensor was continuously monitored by a programmable digital multimeter in the whole measurement process. The sampling interval was set to be 1 s and the test temperature was changed from 100 °C to 300 °C by adjusting the temperature controller of the heating plate. During the measurement, the ambient relative humidity was about 35%–40%.
Figure
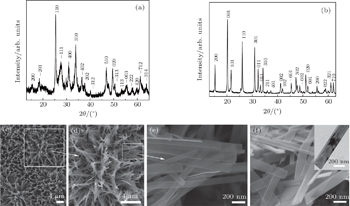 | Fig. 1. XRD patterns and SEM images of (a), (c)–(e) VO2 nanobelts, (b) and (f) V2O5 nanobelts. The inset in panel (f) shows the TEM image of a single bundle of stacked V2O5 nanobelts. |
Figures
Figures
Owing to the chelating and capping effect, in this work, EDTA is chosen to serve as a medium to facilitate the growth of 1D VO2 nanobelts. The concentration of EDTA is found to be crucial in controlling the VO2 morphology. SEM images in Figs.
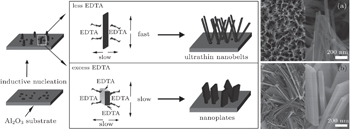 | Fig. 3. Schematic illustration of the in-situ formation mechanism of VO2 samples: (a) ultrathin nanobelts, NH4VO3/EDTA = 6:1, and (b) nanoplates, NH4VO3/EDTA = 2:1. |
The possible growth process of the 1D nanobelts is proposed in Fig.
Gas-sensing properties of the V2O5 nanobelt networks are based on the resistance changes before and after the test gas is injected. To explore the optimum operating temperature, the sensor response of V2O5 nanobelt networks to 250-ppm ethanol gas is first measured at different operating temperatures. Figure
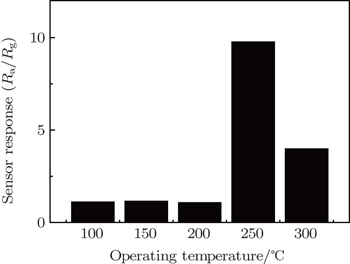 | Fig. 4. Relationship between the sensor response and operating temperature for V2O5 nanobelt networks toward 250-ppm ethanol gas. |
Figure
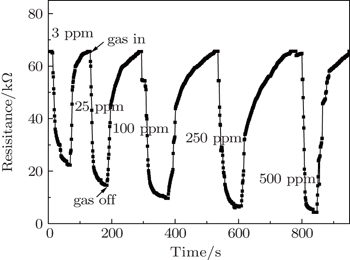 | Fig. 5. Dynamic responses of the gas sensor based on V2O5 nanobelt networks to varying ethanol concentrations at the optimal operating temperature of 250 °C. |
Another point that is important to note in Fig.
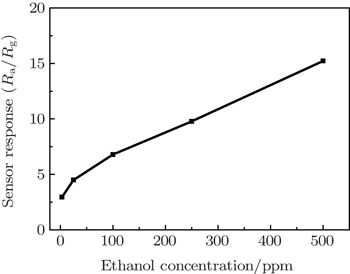 | Fig. 6. Relationship between sensor response and ethanol concentration for the V2O5 nanobelt network sensor at an optimal working temperature of 250 °C. |
It is thought that the efficient utilizations of the surface area and surface accessibility are crucial to maintain the high sensitivity and fast response characteristic of a gas sensor.[25] Thus, the excellent response capability to dilute ethanol gas and fast recovery characteristic could be understood considering the unique structural features of the rough aligned, ultrathin V2O5 nanobelt networks. Ultrathin nanobelt networks can supply a large number of surface active sites facilitating the adsorption of gas molecules due to their high effective surface area. This is favorable for achieving a high gas-sensitivity. Meanwhile, the loose structure of the rough aligned nanobelts, which are assembled by the directly bottom-up growth on the substrate, allows the gas to diffuse agilely in the sensing networks. That is, the gas molecules can be effectively adsorbed on and swept away from the surface of V2O5.[21] As a result, fast response and recovery are achieved.
A novel two-step process, i.e., in-situ inductive nucleation followed by EDTA-mediated hydrothermal growth, is developed to prepare ultrathin and aligned VO2 nanobelts on a substrate. The performed inductive layer is crucial for aligned growth of nanobelts; meanwhile EDTA serves as a capping agent to facilitate the preferential growth of 1D VO2 nanobelts. With mild annealing at 350 °C in air, the as-synthesized ultrathin VO2 nanobelts could transform into ultrathin V2O5 nanobelts. The possible formation mechanism of VO2 nanobelts is proposed by involving the crucial guide role of EDTA in crystal growth. Owing to the good interface performance and gas adsorption–desorption properties, the as-prepared V2O5 nanobelt network exhibits excellent reversibility and stability, high response value, and fast response–recovery characteristic to ethanol gas at its optimal operating temperature of 250 °C. Thus, the presented ultrathin and rough aligned V2O5 nanobelt networks are considered as a promising sensing material in developing an ethanol sensor with low power consumption, excellent sensitivity, and good gas stability.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 |



