† Corresponding author. E-mail:
‡ Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant No. 60877038).
The SnO2/SnO with an orthorhombic structure is a material known to be stable at high pressures and temperatures and expected to have new optical and electrical properties. The authors report a new finding of the infrared laser induced a fast photovoltaic effect arising from orthorhombic tin oxide film with an indirect band gap (∼ 2.4 eV) which is deposited by pulsed laser deposition. The rising time of the photovoltaic signal is about 3 ns with a peak value of 4.48 mV under the pulsed laser beam with energy density 0.015 mJ/mm2. The relation between the photovoltages and laser positions along the line between two electrodes of the film is also exhibited. A possible mechanism is put forward to explain this phenomenon. All data and analyses demonstrate that the orthorhombic tin oxide with an indirect band gap could be used as a candidate for an infrared photodetector which can be operated at high pressures and temperatures.
SnO2/SnO is a wide direct-band gap (3.6 eV) semiconductor with high exciton binding energy (130 meV) at room temperature,[1] and also has an indirect band gap of about 2.6 eV.[2] It is a unique material of significant technological importance [3,4] and is used as transparent electrodes, solar cells, and solid-state gas sensors.[5–8] Under normal conditions, it exists in the form of a crystalline phase known as cassiterite. Another form of SnO2 with an orthorhombic structure is known to be stable only at high pressures and temperatures. The formation of orthorhombic phase SnO2 is intimately tied to a number of important synthesis parameters such as high pressure and temperature.[9,10] By using the pulsed laser deposition (PLD), we discover a simple way to fabricate the orthorhombic SnO2 thin film only by controlling the proper temperature and the ambient oxygen pressure during deposition.[11]
In this paper, we present our new successful fabrication of the orthorhombic tin oxide thin films with an indirect band gap (∼ 2.4 eV) by pulsed laser deposition at an oxygen partial pressure of 1 Pa and a substrate temperature of 450 °C. Under 1064-nm-wavelength pulsed laser irradiating on the sample at room temperature, a fast photovoltaic effect is observed in each of these films. The influence of oxygen vacancies on electrical properties is thought to originate from this photovoltaic effect. We also present the relations between the magnitude of photovoltage and power density of laser pulse and between the photovoltaic signals and laser positions on film. A possible detailed mechanism is put forward to explain this phenomenon. All data and analyses demonstrate that this orthorhombic tin oxide film with an indirect band gap may be a candidate for an infrared detector which can work at high pressures and temperatures.
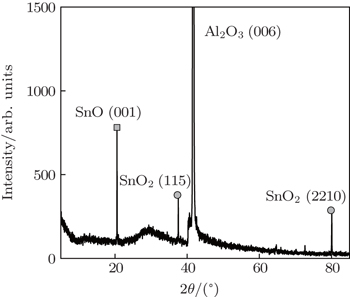
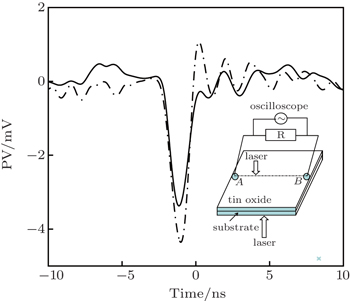
A KrF excimer laser (λ = 248 nm), operating at a repetition rate of 4 Hz with an energy density of about 3 J·cm−2, was used to grow tin oxide thin films on sapphire substrates from a polycrystalline SnO2 target. The distance between target and substrate was kept to be about 5 cm, the substrate temperature was 450 °C and the ambient oxygen pressure was ∼ 0.1 Pa. The film thickness was fixed at 400 nm with an area of 10 mm × 10 mm. The crystalline structure of the deposited SnO2 thin film was measured by x-ray diffraction (XRD) using Cu Kα radiation. The XRD patterns were recorded at a scanning rate of 1 °/s in the 2θ range from 5° to 85°. For the transient photovoltaic measurements, two indium electrodes of 1 mm × 1 mm area were soldered on the two ends of the sample. A mode locked Nd: yttrium–aluminium–garnet (Nd:YAG) laser (pulse duration of 20 ps, repetition rate of 10 Hz) was used to irradiate the sample operating at the wavelengths of 1064 nm and room temperature. A laser spot with a small area of 1 mm in diameter, which was produced by the pulsed laser beam with an energy density of 0.015 J/mm2, perpendicularly irradiated on the film surface. The pulse duration time was ∼ 20 ps. The lateral photovoltage waveform between the indium electrodes was measured and recorded by a 350-MHz sampling oscilloscope terminated into 1 MΩ for open-circuit photovoltage at ambient temperature. The schematic of the experimental set-up for the photovoltage between two electrodes is shown in the inset of Fig.
Figure
To investigate the band gap of tin oxide thin film, we carry out optical absorbance spectrum measurement at room temperature. Figure

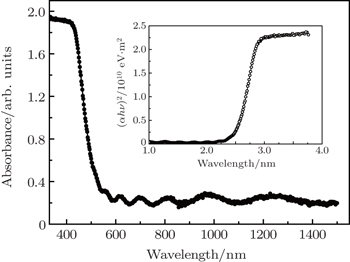 | Fig. 2. Optical absorbance spectrum of tin oxide thin film. The inset shows the plot of α1/2 against hν of tin oxide. |
Typical ultrafast photovoltaic curves (solid and short dash dotted) for the photovoltaic signals are shown in Fig.
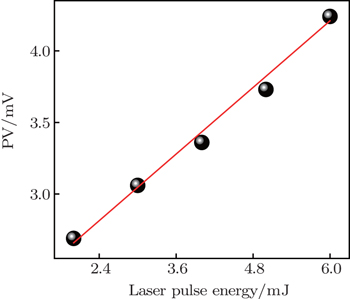
The peak value of the photovoltaic response as a function of power density is plotted in Fig.
In Fig.
 | Fig. 5. Dependence of the peak value of LPV on the position of the laser spot irradiated on the tin oxide surface. |
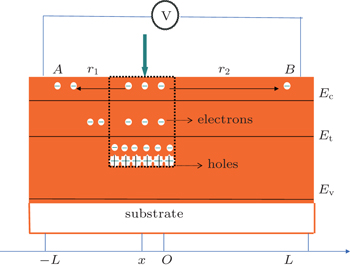
For a better understanding, a quantitative interpretation is given in the ideal one-dimensional model.[19] According to the diffusion equation, the distribution of the light induced electrons in the semiconductor can be calculated from [19]

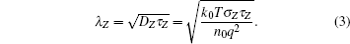
According to the Einstein relation, DZ is the diffusion constant and τZ is the lifetime of the non-equilibrium electrons of the tin oxide layer, σZ is the conductivity of the tin oxide film, and n0 is the area density of electrons at the equilibrium state.
So the peak value of LPV depends on carrier diffusion length λ (or film conductivity σ) significantly. To explain the LPV observed in tin oxide film, we propose the following physical model. Figure
In this study, the indirect band gap (∼ 2.4 eV) orthorhombic tin oxide film is found to exhibit an infrared (IR) laser-induced photovoltaic effect, which is ascribed to the oxygen vacancy energy level, other impurity energy levels and the difference in carrier concentration between the illuminated and the non-illuminated regions. The photovoltage increases linearly with pulsed laser energy in a range from 2 mJ to 6 mJ. When the laser spot scans along the line between the two electrodes, the peak value of LPV changes unusually with the laser position compared with the previously reported results. A possible mechanism is put forward to explain this effect. Our results demonstrate the orthorhombic tin oxide film can also be a candidate for a new type of infrared detector device which can be used at high pressures and temperatures.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 |