† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 11374217, 11135012, and 11375262) and the Joint Fund of the National Natural Science Foundation of China and the China Academy of Engineering Physics (Grant No. 11176020).
Thermodynamic and chemical properties of liquid carbon dioxide and nitrogen (CO2–N2) mixture under the conditions of extremely high densities and temperatures are studied by using quantum molecular dynamic (QMD) simulations based on density functional theory including dispersion corrections (DFT-D). We present equilibrium properties of liquid mixture for 112 separate density and temperature points, by selecting densities ranging from ρ = 1.80 g/cm3 to 3.40 g/cm3 and temperatures from T = 500 K to 8000 K. In the range of our study, the liquid CO2–N2 mixture undergoes a continuous transition from molecular to atomic fluid state and liquid polymerization inferred from pair correlation functions (PCFs) and the distribution of various molecular components. The insulator–metal transition is demonstrated by means of the electronic density of states (DOS).
With the continuing development of fluid science,[1] shock physics, detonation science,[2] and geophysics,[3] the study of material properties under extreme conditions[4] has attracted extensive attention. In the last decade, this has been made possible due to both the increasing accuracy of experimental diagnostics and the expanding computing capabilities. On the experimental techniques side, gas gun,[5] chemical explosives,[6] and pulsed power,[7,8] have been adopted in dynamic compressions, where pressures could reach the megabar region. Among the theoretical models, thermochemical calculations have been developed and concentrated mainly on the dynamic compressions of energetic materials[9] and the equation of state (EOS) of these detonation products, of which N2 and CO2 are usually the main components because of their relatively large negative formation enthalpies.[10] Thermochemical calculations are based on a microscopic description of pair interactions determinated by explosive detonation data.[11] Nevertheless, the EOS predictions would lose their accuracies due to modifications of thermodynamic conditions and chemical composition of the detonation product mixture. Therefore, a variety of direct simulation methods have been developed, such as the path-integral Monte Carlo[12,13] (PIMC) and density-functional theory (DFT) molecular dynamics (MD).[14–17] Up to now, quantum molecular dynamics (QMD)[18,19] has been particularly suited for the study of such chemical processes as ionization, recombination, dissociation, and association of the various atomic species present in the warm dense matter.[18] In spite of the fact that these theoretical simulations in some cases already provided explanatory and predictive results, however, some discrepancies were observed between the results of QMD calculations and the thermochemical predictions.[10,20] Some theoretical results agree well with experiment data only in the case of higher density. It could be argued that DFT would not accurately predict the thermodynamic properties of warm dense matter with some systematic errors due to the fact that dispersion interactions are not taken into account.[21] The van der Waals (vdW) interactions are expected to play a significant role in determining the mechanical properties of some materials. In the study of the thermophysical properties for liquid carbon monoxide and nitrogen (CO–N2) mixture under extreme conditions,[22] we tried to explore the effect of vdW forces on liquid–liquid phase transition in the shock-compressed process, and found that the vdW interaction has a negative contribution to the pressure and tends to reduce the overestimation of the equilibrium volume.
To the best of our knowledge, the pure molecular systems, for exaple, liquid, argon,[23] hydrogen,[24–26] oxygen,[27–31] nitrogen,[32–34] nitrogen oxide,[18,35] and carbon monoxide[18,36] have been intensively studied with a variety of experimental techniques and theoretical simulations. There are only a few first-principles calculations concerning EOS for the liquid mixtures. In the present work, we study thermodynamic properties and chemical decomposition of liquid carbon dioxide and nitrogen (CO2–N2) mixture by using quantum molecular dynamic simulations based on density functional theory including dispersion corrections (DFT-D).[37,38] The liquid nitrogen undergoes a dissociative phase transition above 30 GPa and 7000 K indicated by experiments[39,40] and theoretical calculations.[32,41] Furthermore, as density or temperature increases, metallization can occur through first-order transition accompanied by molecular nitrogen to liquid polymeric phase transition.[42–44] However, the metallization of a compressed liquid mixture remains an open question. Here we study the insulator–metal transition in the liquid mixture by means of the electronic density of states (DOS). The second question from which this work originates is linked to recent results from our QMD simulations on the liquid CO–N2.[22] The data for liquid carbon dioxide[45–48] under shock compressions suggested that shock-induced dissociation transition of CO2 occurred at pressure above 34 GPa and temperature 4500 K along the Hugoniot, while our previous studies show that the decomposition of N2 molecules in liquid CO–N2[22] mixture occurs at temperature T ≈ 3500 K and pressure P ≈ 20 GPa, much lower than those of pure liquid nitrogen.[32,33,39,49–52] Therefore, we will pay particular attention to the constituency of the dissociative phase transition of liquid CO2–N2 mixture.
In the past few years, density functional molecular dynamics (DFT-MD)[24,53,54] has become the most commonly used method to treat fluid systems. Nevertheless, the accuracy of density functional theory (DFT) (Ref. [24]) has often been brought into question, because dispersion interactions are not taken into account.[21] In this study, in order to make up for the effect of dispersion on thermodynamics of the liquid mixture, which is absent in DFT simulations, Perdew–Burke–Ernzerhof augmented by a damped empirical correction for dispersion-like interactions (PBE-D)[55] is adopted. In our work, we employ the Gaussian and plane wave (GPW) method code QUICKSTEP as implemented in CP2K to calculate all of the molecular dynamics trajectories. This method describes the electronic structure by using the density functional theory (DFT) (Ref. [24]) with the generalized gradient approximation (GGA)[56] exchange–correlation functional combined with the Double Zeta Valence with Polarization (DZVP) basis set and the GTH-type pseudopotential.[57,58] Taking the effect of dispersion into consideration, all of the geometry optimizations of isolated molecules are performed with PBE-D. For the nitrogen molecule, the length of triple bond in the molecule is equal to 1.119 Å, comparable to the experimental value of 1.097 Å. For the carbon dioxide molecule, an interatomic distance of C–O of 1.160 Å is calculated in comparison with the experimental value of 1.162 Å, and the molecule is linear. For all these molecules, satisfactory agreement on the geometry of the isolated molecule is achieved, confirming the validity of the method of simulating the intramolecular interactions.
The calculations are performed by a series of volume-fixed unit cells, while keeping the number of molecules of each kind fixed. The supercell in our MD simulation contains 48 molecules (120 atoms) in the case of equimolar binary carbon dioxide and nitrogen (CO2–N2) mixture (24 CO2 and 24 N2 in the form of molecules). Periodic boundary conditions are used in the three directions. The tests of the effects of finite-size are performed with larger cells, each containing 64 molecules, corresponding to densities of 1.80 g/cm3 and 2.89 g/cm3 and a temperature of 2000 K. Compared with the case in our 48-molecule system, the changes in the pressure and internal energy are not significant and smaller than 2%.
We perform fixed-volume molecular dynamics simulations for 112 separate density and temperature points, with seven different specific densities from ρ = 1.80 g/cm3 to 3.40 g/cm3. For each density, the simulation is executed for a temperature range from T = 500 K to 8000 K, and the temperature is increased in steps of ΔT = 500 K. The initial configurations are obtained after a stabilization of the liquid at the same density and a lower temperature T = 300 K, ensuring that all the simulations are started at 1:1 carbon dioxide and nitrogen molecular (CO2–N2) mixture. In order to obtain accurate enough thermodynamic quantities, we let the dynamic simulations last 30 ps, including the system equilibration time for more than 10 ps and the time of calculated properties at least 10 ps, depending on different systems. Also, we obtain the EOS data (P, U, T) and pair correlation functions by averaging over the final 10-ps calculations for all the systems. As an example, the time evolutions of the temperature, the potential energy, and the pressure of the liquid CO2–N2 mixture at T = 2000 K and ρ = 2.20 g/cm3 are shown in Fig.
All quantum molecular dynamics (QMD) calculations are performed within the canonical ensemble (the NVT ensemble) in integration time steps of 0.5 fs, which is applied to all degrees of freedom. The plane-wave energy cutoff radius of 500 Ry (1 Ry = 13.6056923(12) eV) is employed, which is sufficient to obtain a converged energy and force, and a Nosè–Hoover thermostat[59] is used to adjust the system temperature.
We present our QMD simulation results for equilibrium properties of liquid carbon dioxide and nitrogen (CO2–N2) mixture for 112 separate density and temperature points. In the region of our study, the CO2–N2 mixture system undergoes the molecular–atomic fluid state transition and liquid polymerization transition, coinciding with an insulator–metal transition. Thus, it is interesting to examine molecular dissociation and the insulator–metal transitions in studying the thermophysical properties of liquid CO2–N2 mixture under extreme conditions. To check on the accuracy of DFT-D calculations, isotherms at T = 2000 K and T = 3000 K are shown and compared with DFT results calculated by Jean–Bernard Maillet[10] in Fig.
 | Fig. 2. Isotherms at T = 2000 K (squares) and T = 3000 K (circles) for liquid CO2–N2 mixture. Solid symbols correspond to DFT results (Ref. [10]), and empty symbols correspond to our DFT-D results. |
The agreement between DFT-D results and DFT data is good over the whole density range for both isotherms. This is mainly because of the insignificant contribution of van der Waals interactions in those ranges of pressure and temperature. However, as the density and temperature increase, the molecules of the mixture are dissociated and DFT may lose the accurate predictions due to the lack of van der Waals interactions. A more detailed study of the phase transition can be acquired by isochores. The relations of pressure–temperature for different isochores derived from our DFT-D calculations are displayed in Fig.
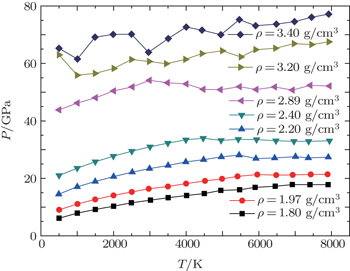 | Fig. 3. Pressure–temperature relations for liquid CO2–N2 mixture along various isochores derived from our QMD/DFT-D simulations. |
As shown in Fig.
At a higher density ρ = 1.97 g/cm3, when the temperature increases up to 6000 K, (ΔP/ΔT)V is slightly negative, which indicates strongly that the mixture is undergoing a phase transition. Remarkably, with the increase of the density, the phase transition takes place at lower temperature, leading to a region of (ΔP/ΔT)V < 0. For example, at a density of 2.40 g/cm3, (ΔP/ΔT)V < 0 is present at a temperature about of 4500 K, while the first drop of the pressure occurs only at 3000 K, when the density increases up to 2.89 g/cm3. At sufficiently high densities of ρ = 3.20 g/cm3 and 3.40 g/cm3, the pressure–temperature (P–T) plane curves each have more than one fold point. This could possibly be caused by a series of continuous phase transitions, such as the dissociations of CO2 and N2 molecules, the recombinations of C, N, and O atoms, the formations of large clusters, and the associations of products under extreme conditions of high pressure and temperature.
As an example, according to the analysis of the P–T curve, at the density of 2.40 g/cm3, the first phase transition of liquid CO2–N2 mixture takes place at a temperature of about 4500 K and pressure up to 34 GPa, which agrees with the conditions of shock-induced dissociation for liquid CO2 reported by Nellis et al.[45] So, it suggests that this phase transition is caused by the decomposition of CO2 molecules. It is not difficult to find that N2 molecules do not dissociate at much lower temperature and pressure (T ≈ 4000 K and P ≈ 20 GPa) than those of molecular nitrogen inferred from experiment[39,49] and predicted in theory[32,33,50–52] (T ≈ 7000 K and P ≈ 31 GPa), as we have mentioned in studying liquid CO–N2 mixture[22] under extreme conditions.
The pair correlation function (PCF)[27,36]
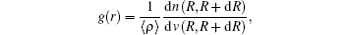
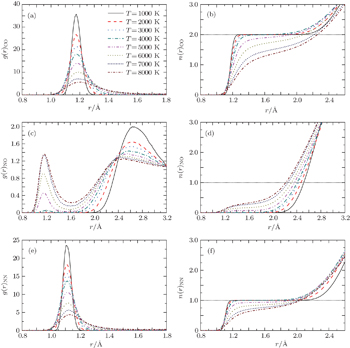
Firstly, we use VMD[60] to analyze the MD trajectories. The dependences of pair-correlation functions g(r)CO, g(r)NO, and g(r)NN, and the associated numbers of neighbors n(r)CO, n(r)NO, n(r)NN for liquid CO2–N2 mixture on temperature at the density of ρ = 2.40 g/cm3 are shown in Fig.
Now we turn our attention to the formation of nitrogen oxides. Figures
Figure
In order to give more direct evidence of structural evolution, we analyze the MD trajectories by using the FINDMOLE code,[61] which can find molecule structures and count the number of molecules in MD simulations trajectories. The time evolutions of initial carbon dioxide and nitrogen, and several important products (dimer N2–CO2, NO, NO2, and N2O) in the liquid mixture are shown in Fig.
Figure
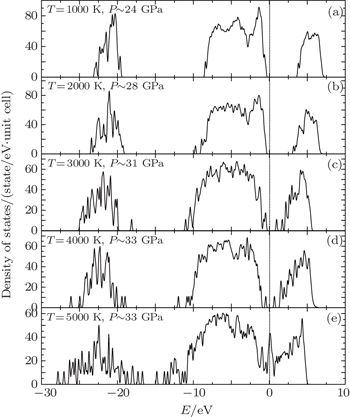
Among the regions of our study, the mixture system undergoes a continuous transformation, accompanied by an insulator–metal transition which is inferred from the electronic density of states (DOS) shown in Figs.
All of the DOS calculations are performed with the GGA/PBE method[56] and the plane-wave cutoff at 500 eV. Figure
 | Fig. 9. Instantaneous configurations of the system at densities of (a) 2.40 g/cm3 and (b) 3.40 g/cm3, respectively, and the corresponding temperatures are both 3000 K during simulations. |
Figures
In this work, we study the thermodynamic properties and chemical decomposition of liquid carbon dioxide and nitrogen (CO2–N2) mixture under the conditions of extremely high densities and temperatures by using the quantum molecular dynamic simulations based on density functional theory including dispersion corrections (DFT-D). The selected pressures range from 6 GPa to 77 GPa, In this range, the liquid CO2–N2 mixture undergoes a continuous transition from molecular to atomic fluid state and liquid polymerization, accompanied by an insulator–metal transition. The liquid mixture molecules begin to dissociate at the temperature above 4000 K, and the corresponding pressure of about 34 GPa, which agrees with the conditions of shock-induced dissociation for liquid CO2. It suggests that the dissociation of N2 molecules do not occur under the N2 decomposition condition in liquid CO–N2 mixture. The main product of nitrogen oxides is NO, which has been testified. Other important products, such as stable dimer N2–CO2, few NO2, intermediate N2O, and the highly transient large clusters of carbon–nitrogen–oxygen are observed.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 | |
| 39 | |
| 40 | |
| 41 | |
| 42 | |
| 43 | |
| 44 | |
| 45 | |
| 46 | |
| 47 | |
| 48 | |
| 49 | |
| 50 | |
| 51 | |
| 52 | |
| 53 | |
| 54 | |
| 55 | |
| 56 | |
| 57 | |
| 58 | |
| 59 | |
| 60 | |
| 61 | |
| 62 |