† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 51472266, 51202286, and 91422303), the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (Grant No. XDB07020100) and the ICDD.
A new layered Cu-based oxychalcogenide Ba3Fe2O5Cu2S2 has been synthesized and its magnetic and electronic properties were revealed. Ba3Fe2O5Cu2S2 is built up by alternatively stacking [Cu2S2]2− layers and iron perovskite oxide [(FeO2)(BaO)(FeO2)]2− layers along the c axis that are separated by barium ions with Fe3+ fivefold coordinated by a square-pyramidal arrangement of oxygen. From the bond valence arguments, we inferred that in layered CuCh-based (Ch = S, Se, Te) compounds the +3 cation in perovskite oxide sheet prefers a square pyramidal site, while the lower valence cation prefers the square planar sites. The studies on susceptibility, transport, and optical reflectivity indicate that Ba3Fe2O5Cu2S2 is an antiferromagnetic semiconductor with a Néel temperature of 121 K and an optical bandgap of 1.03 eV. The measurement of heat capacity from 10 K to room temperature shows no anomaly at 121 K. The Debye temperature is determined to be 113 K. Theoretical calculations indicate that the conduction band minimum is predominantly contributed by O 2p and 3d states of Fe ions that antiferromagnetically arranged in FeO2 layers. The Fe 3d states are located at lower energy and result in a narrow bandgap in comparison with that of the isostructural Sr3Sc2O5Cu2S2.
Layered Cu-based oxychalcogenides have received considerable attention due to their promise in areas including transparent conducting materials,[1–4] thermoelectric materials.[5–7] and photocatalysts,[8] etc. The two-dimensional (2D) nature of these compounds, the (CuCh2)2− layer in particular, is the origin of the interesting transport and optical properties. The wide applications of these compounds are mainly determined by their bandgaps which are tunable. As a narrow band gap semiconductor, BiCuSeO has high mobility originating from the Cu 3d/S 3p antibonding states and low thermal conductivity, making it a promising candidate for commercial thermoelectric applications.[5–7] Meanwhile, the isostructural quaternary oxychalcogenides LnCuOS (Ln = La–Nd) are optically transparent in the visible region and are used as p-type transparent conducting semiconductor.[1] Other promising candidates for wide-band-gap semiconductors are those in which perovskite-type oxide layers containing d0 or d10 metals separate the antifluorite-type [Cu2Ch2]2− layers. For example, Sr2ZnO2Cu2S2, Sr3Sc2O5Cu2S2, and their derivatives have been explored in the context of viable transparent p-type conductors, with bandgaps up to 3.1 eV.[2,3] However, when the d10 metals in the oxide layers of Sr2ZnO2Cu2S2 are replaced by magnetic atoms Co or Mn and the S atoms substituted by Se, a bandgap narrowing occurred and the optical bandgaps of Sr2CoO2Cu2Se2 and Sr2MnO2Cu2Se2 were drastically decreased to 0.068 eV and 0.073 eV, respectively.[9] The reason, however, responsible for this effective bandgap narrowing is still unclear.
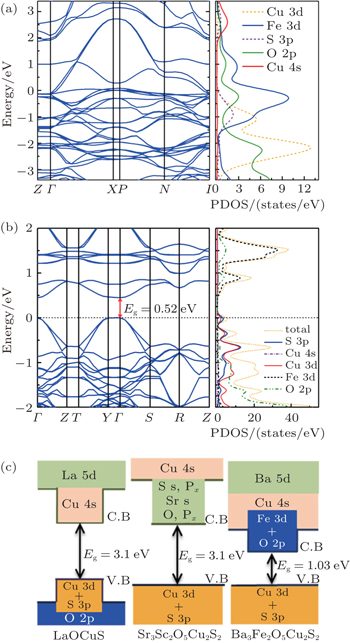
The bandgap widths in Cu-based oxychalcogenides are generally correlated with the electronegativity of chalcogenide, as well as the basal lattice parameter. In LaOCuCh (Ch = S, Se, Te) system, the bandgaps decrease from 3.1 eV to 2.31 eV with reducing the electronegativity of chalcogenide from S to Te.[4] While for a given chalcogenide ions, a decrease of the basal lattice parameter from 4.067 Å to 3.879 Å in LnOCuSe (Ln = Y and La) also results in slight narrowing of the bandgap (from 2.82 eV to 2.58 eV).[10,11] However, the bandgap narrowing observed in Sr2CoO2Cu2Se2 and Sr2MnO2Cu2Se2 is exceptional, since such a drastic narrowing effect was not observed in other nonmagnetic oxychalcogenides systems. A natural speculation then arises that the 3d electrons in the perovskite-type oxide layers might be more or less responsible for such a bandgap narrowing. Therefore, it is desirable to find a new 3d transitional metal oxysulfide to verify this speculation and study the mechanism of bandgaps narrowing in layered Cu-based oxychalcogenides. Here, we report the synthesis and characterization of a new layered Cu-based oxysulfides, Ba3Fe2O5Cu2S2, which is isostructural to nonmagnetic Sr2Sc3O5Cu2S2 without d electrons. Interestingly, compared with the wide bandgap of 3.1 eV in Sr2Sc3O5Cu2S2, the optical bandgap of Ba3Fe2O5Cu2S2 significantly reduced to 1.03 eV. Our theoretical calculations indicated that Ba3Fe2O5Cu2S2 should be a metal without considering the effect of spins, while it becomes a narrow bandgap mott insulator if considering the spin-polarized AFM magnetic interaction. The conduction band minimum (CBM) of Ba3Fe2O5Cu2S2 is comprised of the Fe 3d/O 2p states and is considerably lowered in compared with the isostructural Sr3Sc2O5Cu2S2, which is responsible for the observed bandgap narrowing.
The Ba3Fe2O5Cu2S2 sample was prepared by the reaction of BaO (99.9%), FeO (99.9%), Cu (99.99%), and S (99.999%) powders in stoichiometric ratio. Reagents were mixed together and sealed inside an evacuated quartz tube. The sealed quartz tube was heated slowly to 1123 K and held at this reaction temperature for 24 h before furnace-cooling to room temperature. Powder x-ray diffraction (PXRD) was performed at room temperature using a PANalytical X’pert Pro diffractometer with Cu Kα radiation. Rietveld refinement was performed using the FULLPROF program.[12] DC magnetization M(T) and Resistivity data were measured on a Physical Properties Measurement System (PPMS, Quantum Design) using powders. The diffuse reflectance spectra of the samples were measured on a UV-3600 Plus ultraviolet-visible light-near-infrared (UV-vis-NIR) spectrophotometer over the range 220 nm–2600 nm. Reflectance spectra were converted to absorbance expressed as F(R) using the Kubelka–Munk function. The first principles calculations were performed using the CASTEP program.[13] The generalized gradient approximation (GGA) in the form of the Perdew–Burke–Ernzerhof was chosen to solve the exchange-correlation potentials.[14] The ultrasoft pseudopotential with a plane-wave energy cutoff of 380 eV and a Monkhorst Pack k-point separation of 0.04 Å−1 in the reciprocal space were used for all the calculations.[15] We employed the “LDA + U” (LDA: local density approximation) correction with U = 5 eV for the Fe-3d electrons.[16,17]
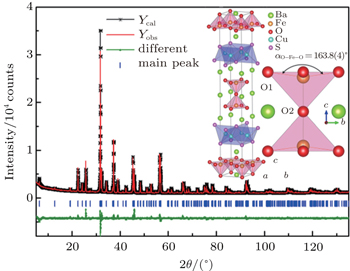
The room temperature PXRD pattern of the Ba3Fe2O5Cu2S2 sample was shown in Fig.
| Table 1. Room-temperature crystallographic data for Ba3Fe2O5Cu2S2. . |
Figure
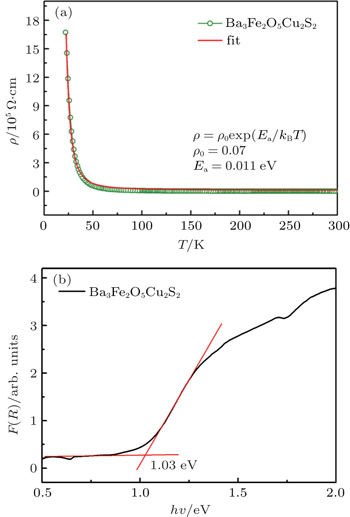
The heat capacity versus T at constant pressure Cp at H = 0 was plotted in Fig.
Figure
The UV-vis-NIR diffuse reflectance spectra of Ba3Fe2O5Cu2S2 were displayed in Fig.
The electrical properties and bandgaps of typical compounds with and without magnetic atoms were summarized and compared in Table
| Table 2. Several typical CuCh-based compounds and their electrical properties. . |
Electronic structures of the titled compound were calculated based on the first principles calculations considering the two cases of the nonmagnetic structure and the spin-polarized structure. A nonmagnetic calculation was first performed where the spins of the magnetic atoms are not considered and the calculated band structure along the high-symmetry k lines was showed in Fig.
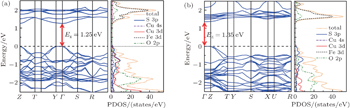
Then, a spin-polarized calculation was performed by using LDA + U method with U = 5 eV. A G-type AFM structure was adopted for Ba3Fe2O5Cu2S2 as reported in Sr3Fe2O5Cu2S2.[34] As shown in Fig.
In summary, a new layered oxychalcogenides compound Ba3Fe2O5Cu2S2 was synthesized and the underlying bandgap narrowing mechanism is revealed by experimental and DFT calculations. Refinement of powder x-ray diffraction data shows that its structure is built up by alternatively stacking [Cu2S2]2 layers and iron perovskite oxide [(FeO2)(BaO)(FeO2)]2− layers along the c axis that are separated by barium ions, with Fe3+ cation locates in a square-pyramidal oxygen coordination geometry. From the bond valence arguments we inferred that in layered CuCh-based oxychalcogenides with perovskite oxide sheets the +3 cationsoccupy square pyramidal sites while the cations with less than +3 locate square planar sites. The optical bandgap of Ba3Fe2O5Cu2S2 is about 1.03 eV, much smaller than the reported nonmagnetic oxychalcogenides. The magnetic susceptibility and transport measurements indicate that Ba3Fe2O5Cu2S2 is an AFM ordering semiconductor with a Néel temperature of 121 K. Electronic structures of a nonmagnetic calculations present metallic behavior, while for a spin-polarized calculation on AFM magnetic structure the results show that Ba3Fe2O5Cu2S2 has a considerable bandgap opened and CBM that are comprised of the Fe 3d/O 2p states lie at much lower energy compared with isostructural Sr3Sc2O5Cu2S2, which result in a bandgap narrowing.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 |