† Corresponding author. E-mail:
‡ Corresponding author. E-mail:
Project supported by the “Strategic Priority Research Program” of the Chinese Academy of Sciences (Grant Nos. XDB05040200 and XDB05010500).
NO3 radicals accumulate during the night, thereby being the most critical night oxidant. Owing to the low concentration and dramatic variation, the detection of atmospheric NO3 radicals is still challenging. In this paper, an LED-based Long Path Differential Optical Absorption Spectroscopy (LPDOAS) instrument is developed for measuring the atmospheric NO3 radicals. This instrument is composed of a Schmidt–Cassegrain telescope, a combined emitting and receiving fiber, and a red LED equipped with a thermostat, and has a center wavelength of 660 nm, covering the NO3 strongest absorption peak (662 nm). The influence of LED temperature fluctuations is discussed. The temperature of the LED lamp with a home-made thermostat is tested, showing a stability of ±0.1 °C. The principle and fitting analyses of LED-LPDOAS are presented. A retrieval example and a time series of NO3 radical concentrations with good continuity for one night are shown. The detection limit of NO3 for 2.6-km optical path is about 10 ppt.
NO3 radical is a significant species during the night, affecting the formation of secondary organic aerosol (SOA) at night.[1–3] It is also the most pivotal oxidation at night, involved in the removal process of NOx and VOCs,[4–6] which is directly related to atmosphere cleanliness. The high activity, low life expectancy and extremely low density make atmospheric NO3 radicals difficult to measure.[7]
A number of different techniques based on optical absorption in the visible region,[8–14] matrix isolation electron spin resonance (MI-ESR),[15] laser-induced fluorescence (LIF),[16] and chemical ionization mass spectrometry (CIMS)[17] have been used to measure NO3 successfully. In recent years, there were three optical techniques commonly used: DOAS,[8,9] Cavity Ring-Down Spectroscopy (CRDS),[10,11] and Cavity-Enhanced Absorption Spectroscopy (CEAS).[12–14] DOAS is the most widely used analytical technique for monitoring atmospheric NO3 radicals due to being a non-contact measuring method, avoiding the influences of sampling, chemical changes and other factors on the measurement. In 1980, Platt et al. first used LPDOAS instrument to detect atmospheric NO3 radicals in polluted areas.[18] Traditional DOAS device uses a xenon lamp as a light source. Xenon lamp is a heat source with band width and powerful light. In contrast, LED light has lighter weight, smaller size, lower power consumption, less heat, more efficient and stable spectrum with no security risks, and because of the narrow-band spectral characteristics, there is no need for filters which will simplify the system.[19] The employment of LED in LPDOAS instruments has great advantages in terms of portability and miniaturization.[20–22] In 2006, Kern et al. first used a red LED with a 625-nm center wavelength as the light source of LPDOAS to measure atmospheric NO3 radicals with a 6-km optical path and the average detection limit was 16 ppt. However, intrinsic thermal drift of LED light will reduce the stability of the light source and if the spectrum shape changes, narrowband variation may occur and interfere with absorption structures of the target gas.[19] Besides, the absorption of NO3 at 623 nm is weaker than that at 662 nm, which was found to lead to lower detect sensitivity.[23]
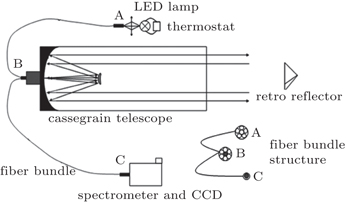
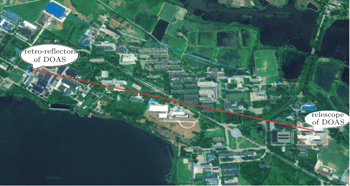
In the present paper, a 660-nm LED is chosen as our light source and its temperature drift characteristics are tested, and a shift of as much as 0.121 nm/°C at the center wavelength and its influence on the fitting results are found. In order to improve the feasibility of the LED-LPDOAS device for detecting atmospheric NO3 radicals, we mount an LED lamp with a self-developed temperature control device, and test the stability. The LED lamp is combined with a Schmidt–Cassegrain telescope and a Y-fiber bundle.[24] A field experiment for observing the atmospheric NO3 radicals is carried out to examine the instrument.
Differential Optical Absorption Spectroscopy (DOAS) based on Lambert–Beer absorption law uses the characteristic absorption of trace gas in light radiation to quantify the concentration.[15] Light I0(λ,L) emitted by a light source transmits through the atmosphere, and finally is received by a detector, with denoting the received light as I(λ,L), relationship between I(λ,L) and I0(λ,L) is given by

As shown in Fig.
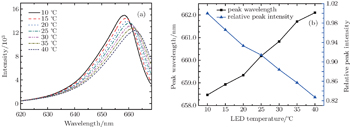
The LED light spectra are taken at different temperatures to test the temperature drift characteristics. The temperature is adjusted by a self-developed temperature control device (stability: ±0.1 °C) in a range from 10 °C to 40 °C, while operating current is set to be 700 mA (stability: ±1 mA). When measuring light not through the atmosphere, place a diffuse reflector in front of the transceiver side B vertically to obtain the original lamp spectrum without atmospheric absorption: the light exiting from the outer core of side B is diffusely reflected, a portion of reflection is received by the inner core of side B and then transmitted to the spectrometer. Figure
In order to clarify the influence of LED temperature fluctuation on data fitting, two groups of reference spectra taken under different temperature conditions are fitted to the same differential optical density (see the specific fitting process in Subsection 2.4). Figure
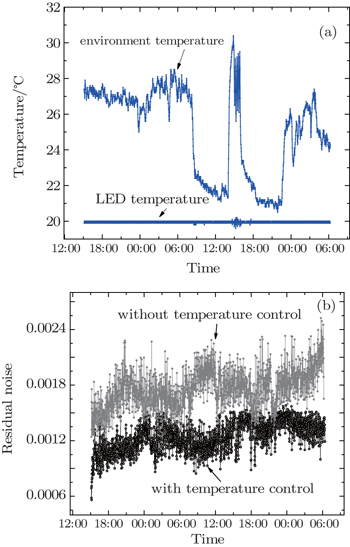
In order to ensure the stability of the light source, an LED test lasting two nights and one daytime is conducted. During the experiment, LED light operating current is set to be 700 mA, the temperature of LED with controller is set to be 20 °C and ambient temperature is adjusted by air-conditioning in the laboratory. Continuous light spectra are taken for 39 h while the temperature data are recorded by the temperature control device every one minute. All spectra are corrected for offset and dark current before being divided by the first spectrum, then high-pass filtered, and the residual noise of each processed spectrum is recorded within the NO3 fitting channel (654 nm–670 nm). Figure
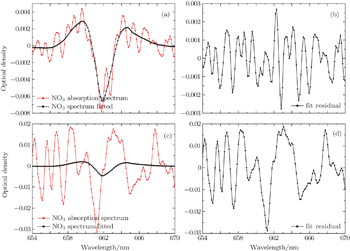
In a first step, night-time atmospheric spectra are corrected by subtracting the offset and background light spectra, removing slow change through using a high-pass filter, and smoothing the spectrum through a low-pass filter to obtain the differential optical density. Secondly, the literature high-resolution absorption cross sections of NO3,[25] NO2,[26] and H2O (from the HITRAN database)[27] are convoluted with the instrument function to be suitable for the apparatus and multiplied by the optical path to obtain the reference differential absorption cross-section. Figure
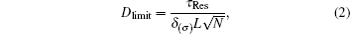
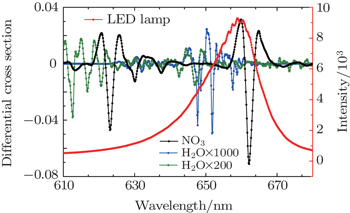
Studies[19] show that three spectra(c1, c2, c3) only with different vapor concentrations and the vapor concentrations of which follows the order: c1 < c2 < c3, when fitting c1 and c3 to c2, the water vapor structure can be deducted well according to the quite small residual structures. The daytime NO3 concentration is very low due to the photolysis, so the daytime atmospheric absorption spectrum (at solar zenith angle less than 80°) can act as a reference spectrum after using the same process to deduct water vapor and LED lamp structure. Thirdly, in the actual evaluation of NO3, two daytime spectra taken at 1.5 h before sunset and after sunrise are performed with the same procedure of the night-time atmospheric spectra, to obtain the reference spectra. Fourthly, the reference spectra with NO3, NO2, and H2O reference differential absorption cross-sections are fitted to the differential optical density to obtain the concentration of NO3 radicals. The whole retrieval procedure is implemented by DOASIS software which is developed by the Institute of Environmental Physics in Heidelberg University, Germany.[28]
As shown in Fig.
Figure
 | Fig. 7. (a) Fitting spectrum of NO3, (b) fitting residual spectrum, and (c) fitting vapor absorption spectrum. |
Figure
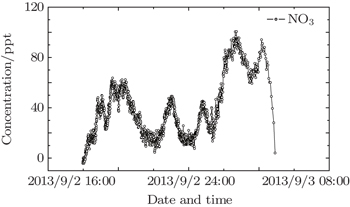
NO3 radical is a critical atmosphere species in the night and its precise observation is particularly important for studying the night atmospheric chemistry. Owing to the continuous development of the materials, LED light covers a wider waveband. The employment of LED in optical instruments has become a trend in recent years. In addition, modern LED has a higher power that can maintain sufficient intensities to obtain a higher temporal resolution. This study is to use Schmidt–Cassegrain telescope, transceiver optic fiber and 660-nm stable LED light equipped with self-developed temperature control device to observe atmospheric NO3 radicals based on LP-DOAS, and the detection limit is about 10 ppt with a 2.16-km optical path. This article provides a tool for studying night atmosphere NO3 radicals.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 |