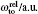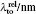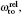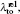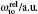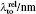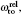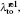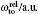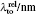† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 11304063 and 11174066) and the Youth Foundation of Liaoning Normal University, China (Grant No. LS2014L002).
An approximation formula is developed to determine the tune-out wavelengths for the ground states of the alkaline-metal atoms lithium, sodium and cesium from the existing relativistic reduced matrix elements and experimental energies. The first longest tune-out wavelengths for Li, Na, and Cs are 670.971 nm, 589.557 nm, and 880.237 nm, respectively. This is in good agreement with the previous high precise results of 670.971626 nm, 589.5565 nm, and 880.25 nm from the relativistic all-order many-body perturbation theory (RMBPT) calculation [Phys. Rev. A
The realization of mixture of trapped ultracold atomic gases in optical lattice is one of the most important improvements in the experimental side in recent years.[1–5] This opens new paths toward the formation of ultracold diatomic molecule and some other targets that the physicists have been seeking for a couple of years.[6–10] The atom can be trapped in the optical lattice by the optical dipole force and will experience an energy shift due to the ac stark effect. Both effects are proportional to the dynamic polarizability of the atom and can be cancelled at certain wavelength where the dynamic polarizability of the atom goes to zero. This means that the atom is unaffected by the presence of the electromagnetic field and also can be released from the trap simply while the other atom in the mixture are still strongly trapped. Such wavelength is termed as the “tune-out wavelength”[11,12] and has attracted much attention recently.
A number of experimental measurements on the determination of the tune-out wavelengths have been performed in the past few years. Since the measurement is a null experiment, it is not subject to the strength of an electric field or the intensity of a laser field. The tune-out wavelengths for the Rb and K atoms have been measured by different groups.[13–15] Holmgren et al.[14] measured the longest tune-out wavelength of potassium to an uncertainty of 1.5 pm by using an atom interfermeter. This is more accurate than the uncertainty of 3 pm which is performed by using state-of-the-art atomic theory.[12] Some tune-out wavelengths for rubidium have also been measured through the diffraction of a Bose-Einstein condensate off a sequence of standing wave pulses.[13] On the theoretical side, the most reliable prediction of the tune-out wavelength for the alkaline-metal atoms is the relativistic all-order many-body perturbation theory (RMBPT) calculation.[12]
In this paper, we present an estimate of the longest tune-out wavelengths for the alkaline-metal atoms: lithium, sodium, and cesium using a formula which is obtained through the analysis of an exact non-relativistic calculation of the tune-out wavelengths for lithium and sodium. This non-relativistic calculation of the tune-out wavelengths are computed using the configuration interaction plus core polarization (CICP) method in which a semiempirical potential is adopted to describe the polarization effect between the core and valence electrons. This method has been successfully applied to the description of many one- and two-electron atoms.[16–22] The detailed analysis of the exact CICP calculation shows that the tune-out wavelengths are determined by explicit calculation of the dynamic polarizability at a series of ω values and they all tend to be close to the transition energies of np states for the alkaline-metal atoms. The fine structure split is not considered in CICP calculation and thus it cannot be used to give the accurate values of tune-out wavelengths. However an approximation formula can be built since that the CICP calculation shows that the values of the tune-out wavelengths are mostly determined by the data of only a few transitions. Using this formula, one can use the existing experimental and theoretical transition information to predict the tune-out wavelengths for the alkaline-metal atom.
The present relativistic estimate of the three longest tune-out wavelengths for lithium and four longest wavelengths for sodium are given in this paper along with the related non-relativistic calculation as a validity. Due to the strong relativistic effect, we do not give the CICP calculation about Cs, but only present the relativistic estimates of the five longest tune-out wavelengths in Section 3.
The details of the non-relativistic CICP method have been given elsewhere[17,18] and here we only briefly outline this method. The CICP method was a frozen-core procedure. The core electron was represented by a Hartree–Fock wavefunction that was calculated exactly using Slater-type orbitals (STOs). The effective Hamiltonian for the valence electrons was essentially a fixed core Hamiltonian with the addition of semiempirical potentials to allow for the polarization interaction with the core.[23] The direct and exchange interactions of the valence with the Hartree–Fock core electrons were computed without approximation. The semiempirical polarization potential was tuned so that they reproduced the binding energies of the ns ground state, the np and nd excited states.[23] The effective Hamiltonian was diagonalized in a very large orbital basis of Laguerre-type orbitals for each l value due to the superior linear dependence properties. The semiempirical core polarization potential Vp has the functional form:
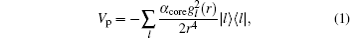
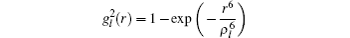
A good test of the CICP method comes from the tabulation of the energy in Table
| Table 1. Theoretical and experimental energy levels (in Hartree unit) for some of the low-lying states of alkaline-metal atoms. The experimental data were taken from the NIST tabulation.[24] The experimental energies for the doublet states are averages with the usual (2J + 1) weighting factors. . |
The dynamic polarizability is defined as
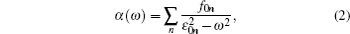
| Table 2. Static dipole polarizability for the ground states of the lithium, sodium and cesium atoms. Numbers in brackets represent the uncertainties in the last digits. All values are in atomic units. . |
Table
| Table 3. Breakdown of contributions to the static polarizability and the dynamic polarizabilities at different tune-out wavelengths, λto, for lithium and sodium. The adopted experimental transition energies are also listed. . |
As can be seen from Table
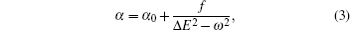
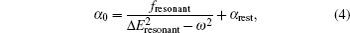
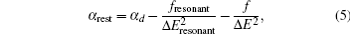
| Table 4. Tune-out frequencies, ωto, and wavelengths, λto, for the four tune-out wavelengths of Li from a non-relativistic CICP calculation. The contribution to the polarizability at the tune-out frequency due to the resonance transition is given. The transition energies are taken from the NIST tabulation.[24] The adopted CICP values of Sresonant, fresonant, and αd are 16.5124, 0.747538, and 164.205 a.u. respectively. . |
| Table 5. Tune-out frequencies, ωto, and wavelengths, λto, for the four tune-out wavelengths of Na from a non-relativistic CICP calculation. The contribution to the polarizability at the tune-out frequency due to the resonance transition is given. The transition energies are taken from the NIST tabulation.[24] The adopted CICP values of Sresonant, fresonant, and αd are 18.6619, 0.961838, and 162.801 a.u. respectively. . |
Based on the analysis of the non-relativistic calculation and the fact that the fine structure split of the energy levels is not considered, the present non-relativistic calculational methodology cannot be applied to the determination of the accurate tune-out wavelengths. However, equation (
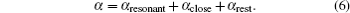
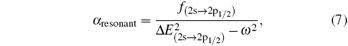
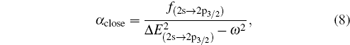
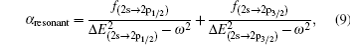
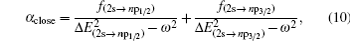
Similar form is adopted for the sodium atom with the 2pj state replaced by the 3pj state, and for cesium with the 2pj state replaced by the 6pj state.
All the fundamental information adopted here to make an estimate of the tune-out wavelength for lithium, sodium and cesium is listed in Table
| Table 6. The experimental transition energies[24] and oscillator strengths used in the calculation. The oscillator strengths are calculated using the reduced matrix elements from the CCSD(T)[33] and SD[25] calculation. . |
| Table 7. Tune-out frequencies, |
| Table 8. Tune-out frequencies, |
The element Cs has been the subject of various theoretical calculations and experiments in atomic clock research.[34,35] The value of tune-out wavelength for Cs could be very useful for experimentalist. The measurements of tune-out wavelengths are also used to test the precision of the excited-state matrix elements which are difficult to measure or calculate. Due to the large correlation corrections, the transition matrix elements are hard to calculate accurately. The measurement of 6s–7pj transition in Cs has been performed in Ref. [36]. The longest tune-out wavelengths and the contributions to the polarizability at the tune-out frequency for Cs are given in Table
| Table 9. Tune-out frequencies, |
An estimate of the uncertainties for the first longest tune-out wavelength of Li, Na, and Cs can be made through Eq. (
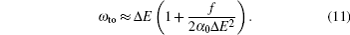
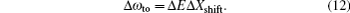
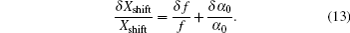
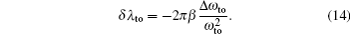
Another useful parameter for the measurement of tune-out wavelength is the energy window Δω. We suppose that the condition for determination of the tune-out wavelength is that the polarizability be set to zero with an uncertainty of ±0.1 a.u. The photon energy range at which the dynamic polarizability is 0 ± 0.1 a.u. is termed as the energy window Δω,
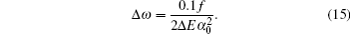
Using a similar method, we could also obtain the uncertainties for other longest tune-out wavelengths. The uncertainties for the second and third longest tune-out wavelengths of lithium are 0.013 nm and 0.009 nm, respectively. The corresponding energy windows are 1.49 × 10−6 a.u. and 2.71 × 10−6 a.u. For sodium, the uncertainties for the second, the third, and the fourth tune-out wavelengths are 0.063 nm, 0.0001 nm, and 0.013 nm. The corresponding energy windows are 1.74 × 10−6 a.u., 6.26 × 10−10 a.u., and 5.22 × 10−7 a.u. For the cesium atom, the uncertainties for the second and third longest wavelengths are 0.559 nm and 0.748 nm which are relatively larger since the matrix elements are more difficult to calculate precisely. The corresponding energy windows are 7.49 × 10−8 a.u. and 1.16 × 10−7 a.u.
The longest tune-out wavelengths for the lithium, sodium and cesium atoms are computed by an approximation formula. The accuracy of the calculation is tested by comparing with the all-order RMBPT calculation.[12] The first longest tune-out wavelengths for Li, Na, and Cs are 670.971 nm, 589.557 nm, and 880.237 nm, respectively. The agreement of the first longest tune-out wavelength is at the 0.001-nm level. Some other longest tune-out wavelengths are also calculated, and there is a reasonable agreement with the RMBPT results.[12] The wavelengths near some resonances which are in the ultraviolet are not calculated, since they are difficultly detected in most laboratories. The uncertainties of the predicted tune-out wavelengths are derived from the difference among the existing transition matrix elements. An parameter termed as the energy window of tune-out wavelength is developed as an indication of the technology required to measure these wavelengths. The longest tune-out wavelengths for lithium and sodium are difficult to measure with existing technology due to their too narrow energy windows while the first three tune-out wavelengths for atomic cesium and the tune-out wavelengths for lithium positioned at 324.161 nm and 274.883 nm and for sodium positioned at 331.839 nm are more feasible choices.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 |