† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant Nos. 11174242, 11204265, 11404278, 11147007, and 11274151), the Natural Science Foundation of Jiangsu Province, China (Grant No. BK2012248), and the Scientific Research Foundation of Yancheng Institute of Technology, China (Grant No. KJC2014024).
A new family of superhard carbon allotropes C48(2 i + 1) is constructed by alternating even 4 and 8 membered rings. These new carbon allotropes are of a spatially antisymmetrical structure, compared with the symmetrical structures of bct -C4, Z -carbon, and P -carbon. Our calculations show that bulk moduli of C48(2 i + 1) are larger than that of c -BN and smaller than that of cubic-diamond. C48(2 i + 1) are transparent superhard materials possessing large Vicker hardness comparable to diamond. This work can help us understand the structural phase transformations of cold-compression graphite and carbon nanotubes.
Superhard materials play an important role in fundamental science and technological applications, and thus they have been widely used in numerous industrial fields, such as cutting, drilling tools, polishing, and coatings for surface protecting. [ 1 , 2 ] Among them, diamond is the hardest known material because of the nature of their strong sp 3 -hybridized covalent bonding, and has been used to fulfill most of the above mentioned industrial needs. Most of the natural diamonds are formed by carbon-containing minerals under the conditions of high temperature and pressure in the Earth’s mantle. Therefore, amorphous carbon, graphite, fullerene, and carbon nanotubes (CNTs) are considered as the potential precursors to synthesize diamond-like materials in high pressure experiment. For example, graphite can be converted into the hexagonal (or cubic) diamond at pressures above 15 GPa and high temperature (1600–2500 K). [ 3 ] In contrast, cold compression of graphite, [ 4 – 14 ] single-walled and multi-walled CNTs [ 15 – 21 ] result in superhard carbon allotropes, which exhibit superior mechanical performance with the ability to indent single-crystal diamond [ 9 , 15 ] and differ from the crystal structure of hexagonal and cubic diamond. Consequently, several hypothetical structures are proposed to shed light on the superhard mechanism. [ 22 – 30 ] The experimental crystal structures of cold-compressed phases of CNTs and graphite are currently still under debate. Therefore, it is essential to examine its intrinsic structure to understand the unexpectedly high indentation hardness of the cold-compressed phases of CNTs and graphite.
When we pay more attention to the previously proposed allotropes, the bct -C4 phase and Z -carbon interest is due to its unique structure. [ 25 – 28 ] Interestingly, all of them can be constructed through sliding and buckling of the graphene sheets. Meanwhile, the bct -C4 phase can be observed at the interface of Z -carbon. The crystal structure of bct -C4 is shown in Fig.
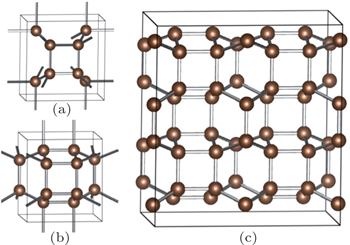 | Fig. 1. (a), (b) Crystallo-graphic structures of the bct -C4 and the proposed C48 carbon, and (c) 2×2×1 supercell structure of C48 phase. |
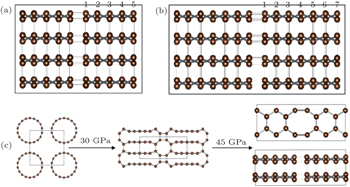 | Fig. 2. (a), (b) 2D projections of the 2×2×1 suppercell of C48(5) and C48(7), and (c) high-pressure phase transition of (9,0) CNTs under different compressions. |
In the present work, we report on a new family of sp 3 -hybridized carbon allotropes by discussing the topological stacking of carbon atomic chains consisting of even 4 and 8 membered ring patterns. From Fig.
The calculations were carried out by using density functional theory as implemented in the Vienna ab initio simulaiton package (VASP) [ 32 ] with the projector augmented-wave method employed to describe the interaction between the nucleus and valence electrons. The general gradient approximation (GGA) of the Perdew Burke Ernzerhof (PBE) parameterization [ 33 ] was adopted to describe the exchange and correlation function. A plane-wave basis set with a cutoff energy of 700 eV was used and Monkhorst–Pack [ 34 ] k meshes were chosen to ensure that all the enthalpy calculations are well converged to better than 1 meV/atom. Forces on the ions were calculated through the Hellmann–Feynman theorem allowing a full geometry optimization.
Regarding the new structural models we explored, the precisions of the electronic structures we forecast are mainly determined by both the accuracy of the lattice optimization and the corresponding Thomas–Fermi screening length averaging scheme that is for sX-LDA calculations. In the CASTEP code, the Thomas–Fermi screening length ( k TF ) of sX-LDA was determined by evaluating the dynamical average charge density within our newly predicted structures. [ 35 ] We took this feature to accomplish the prediction of the electronic structures of the newly determined structures. This is currently unavailable in VASP but we still rely on VASP-predicted geometry structures due to their perfect balance between efficiency and accuracy. Phonon calculations have been carried out by using a supercell approach as implemented in the PHONOPY code. The XRD patterns for various structures were performed by the Materials Studio package.
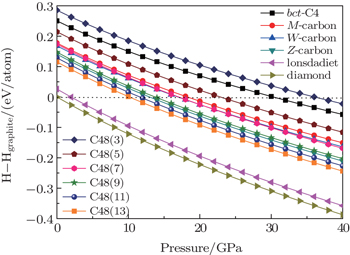
In order to investigate the relative stabilities of C48(2 i + 1) phases, the calculated enthalpy difference at zero temperature with respect to graphite for different allotropes are compared in Fig.
Based on the sX-LDA method, the electronic band structure is calculated at zero pressure. E g = 5.476 eV and E g = 6.11 eV are obtained respectively for cubic and hexagonal diamond, which match well with the experimental results. [ 36 ] It is shown that the C48(3) carbon is a wide gap insulator with energy gap E g = 5.647 eV, which is higher than that of cubic diamond and samller than that of hexagonal diamond. Analyses of electron localization functions (ELFs) (see Fig.
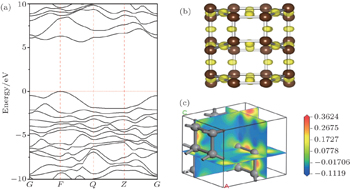 | Fig. 4. (a) Calculated electronic band structure at zero pressure; (b) calculated ELF isosurface at ELF = 0.9; and (c) charge density difference for C48(3). |
| Table 1. Space group, lattice parameters (LP), densities ( D : g·cm −3 ), band gaps ( E g : eV), bulk moduli ( B 0 : GPa), shear moduli ( G : GPa), Young’s moduli ( Y : GPa), Poisson’s ratios ( ν ), and Vicker’s hardness for bct -C4, M -Carbon, W -Carbon, Z -Carbon, Lonsdadiet, diamond, and C48. . |
The mechanical properties, such as bulk modulus, shear modulus, Young’s modulus, Poisson’s ratio, and Vicker’s hardness for bct -C4, M -carbon, W -carbon, Z -carbon, hexagonal diamond, cubic diamond, C48(2 i + 1) are summarized in Table
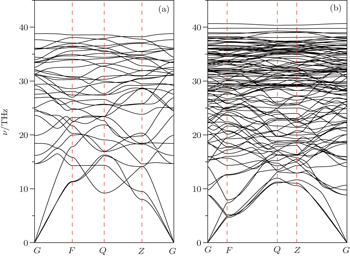
The stability of a structure cannot be determined exclusively by comparing enthalpy since the structure might be subject to dynamic instabilities. Therefore, phonon dispersion curves for C48(2 i + 1) are further calculated by the supercell method. In the present work, we focus on C48(3) and C48(5). C48(3) and C48(5) can be constructed by connecting malposed hexagonal to hexagonal diamond along the z axis and a grain boundary between the hexagonal diamond is observed. The primitive cell of the C48(3) phase contains 12 atoms, giving 36 phonon branches. Around the Γ point, three phonon dispersion curves are observed and no imaginary phonon frequencies are found in the whole brillouin zone (see Fig.
Finally, the XRD patterns of bct -C4, M -carbon, W -carbon, Z -carbon, hexagonal and cubic diamond, C48(2 i + 1) phases have been simulated for instructing the experiment, by using 0.3329 Å radiation. [ 39 ] At zero pressure, the simulated intensive peak positions and relative intensities of cubic and hexagonal diamond match the experimental results very well. As is well known, each phase has its own characteristic peaks, which correspond to different crystal planes. The XRD patterns for C48(2 i + 1) phases are shown in Fig.
In this work, we perform a comprehensive study on the lowest-enthalpy carbon phases and the phase transformation mechanisms for cold-compressed CNTs and graphite. Our calculations reveal that the phase transformation initiates and proceeds along the pathways with lowest enthalpy toward a new family of sp 3 -hybridized C48(2 i + 1) phases. The cold-compressed carbon phases, constructed from CNTs and graphite, may consist of C48(2 i + 1) and previously proposed bct -C4, M -carbon, W -carbon, Z -carbon, P -carbon, S -carbon, R -carbon, and X -carbon. It would be reasonable to expect that the cold-compressed phase of CNTs and graphite can be interpreted as a mixture of several of the proposed allotropes. The present results provide an excellent account for the experiment on the cold-compressed CNTs and graphite, they also provide an insight into the understanding of high-temperature phase transformation pathways for carbon structures.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 | |
| 24 | |
| 25 | |
| 26 | |
| 27 | |
| 28 | |
| 29 | |
| 30 | |
| 31 | |
| 32 | |
| 33 | |
| 34 | |
| 35 | |
| 36 | |
| 37 | |
| 38 | |
| 39 |