† Corresponding author. E-mail:
Project supported by the National Natural Science Foundation of China (Grant No. 11034002), the National Basic Research Program of China (Grant No. 2011CB921602), and Qing Lan Project, China.
Hyperfine structures of ICl in its vibronic ground state due to the nuclear spin and electric quadruple interactions are determined by diagonalizing the effective Hamiltonian matrix. Furthermore, the Stark sub-levels are precisely determined as well. The results are helpful for electro-static manipulation (trapping or further cooling) of cold ICl molecules. For example, an electric field of 1000 V/cm can trap ICl molecules less than 637 μK in the lowest hyperfine level.
We know less about the hyperfine structures of molecules than the rotational structures, because the hyperfine structures are much more complicated and thus hard to model in theory, and they are also hard to observe in experiment due to Doppler broadening. However, thanks to the developments of molecular cooling (see, for example, Stark deceleration of chemically stable molecules, [ 1 – 3 ] diatomic alkali ultracold molecules from laser-cooled atoms, [ 4 – 7 ] and direct laser cooling of molecules [ 8 ] for details), single mode laser technique, and optical comb technique, the molecular hyperfine structures can be investigated precisely at present. Therefore, the experimental data can verify and improve the theory, which makes it possible to precisely study the weak interactions of the nuclear spin and the nuclear electric quadruple moment with electrons surrounding. In addition, the external field effects of the molecular hyperfine structures can be observed and modeled as well. Unique and complex molecular hyperfine structures together with their external field effects provide many opportunities for sensitive probing of fundamental physics and for related applications. External fields have been utilized to manipulate molecules at hyperfine level [ 4 ] and can be used for further cooling cold molecules at about 10 mK to ultracold molecules below 1 μK. [ 9 ]
The electronic ground state of the iodine chloride (ICl) molecule is of 1 Σ symmetry, so its rotational structures have neither Zeeman effect nor Stark effect. Its rotationally resolved spectra can be used as frequency standards for optical frequency calibration in the near infrared region instead of the iodine spectra. The spins of 35,37 Cl atoms are both 3/2 and the spin of 127 I atom is 5/2, and both atoms of ICl have relatively large electric quadruple moments, which result in complicated hyperfine structures. Therefore, ICl is a good candidate to fully understand the hyperfine interactions. Additionally, our recent study suggests that ICl can also be Stark decelerated assisted by a laser beam to the temperature of about 20 mK, which makes it possible to experimentally study the hyperfine structures together with their external field effects. The ICl molecule is the first diatomic molecule whose pure rotational spectrum is investigated by microwave spectroscopy, and its molecular constants B e and α e have been accurately determined. [ 10 , 11 ] Townes et al. obtained the nuclear electric quadruple moments, eQq 1 and eQq 2 , of the two atoms of I 35 Cl by analyzing the microwave spectra. [ 12 ] Thereafter, Slotterback et al. fully analyzed the hyperfine spectrum of I 35 Cl, [ 13 ] and Durand et al. determined the electric dipole moment ( μ ) of ICl. [ 14 ]
The hyperfine structures and their Stark effects of ICl in the rovibronic ground state are studied theoretically by diagonalizing the Hamiltonian matrix in the present work. Our results show that an electrostatic field can be employed for trapping and further cooling of cold ICl molecules.
The Hamiltonian of a molecule in an electric field consists of five parts: electron, vibration, rotation, hyperfine, and Stark parts. When studying the structure of a rovibronic state, the first two parts are constant and thus can be omitted in the Hamiltonian, and then the Hamiltonian can be written as [ 15 , 16 ]
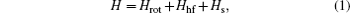

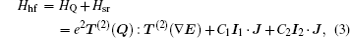
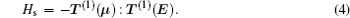
In the weak field limit, J , I 1 , F 1 , I 2 , F , and M F are good quantum numbers to describe the energy levels, and the coupling sequences are
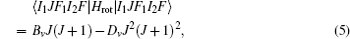
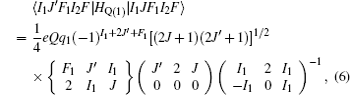
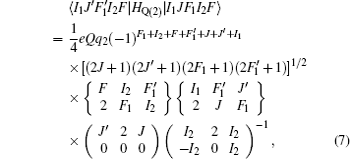
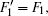
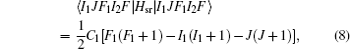
The Stark part of the Hamiltonian matrix is [ 22 ]
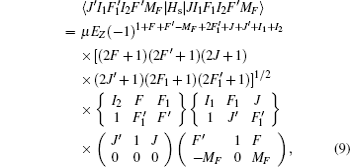
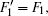
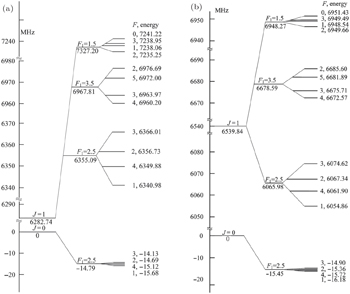
The hyperfine structures of the vibronic ground state of ICl are studied in this section. The spin of 127 I atom is 5/2, and the spins of 35,37 Cl atoms are both 3/2. As described in Section 2, we diagonalize the Hamiltonian matrix on the basis of | JI 1 F 1 I 2 F 〉 to obtain the hyperfine levels of J = 0 and 1 rotational states. The molecular constants adopted in the present calculation are listed in Table
| Table 1. Molecular constant (in MHz) of the v = 0 level in the X 1 Σ state of I 37,35 Cl. . |
When the Stark interaction of Eq. (
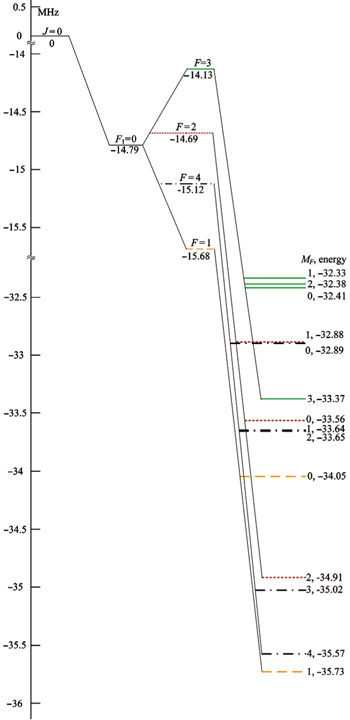 | Fig. 2. Stark splitting of the | J = 0, F 1 = 0, F 〉 hyperfine levels in the vibronic ground state of I 35 Cl at an applied electric field of 1000 V/cm. |
Figure
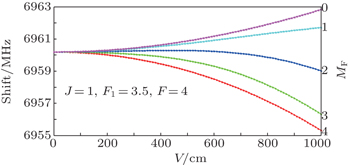 | Fig. 3. Stark shifts of the | J = 1, F 1 = 3.5, F = 4〉 hyperfine level in the vibronic ground state of I 35 Cl varying with the applied electric field. |
Recently, we proposed a laser-assisted Stark deceleration of ICl scheme, and it would most probably decelerate ICl to the equivalent temperature of about 20 mK. Therefore, if we trap and further cool the decelerated ICl via evaporation cooling method [ 9 ] with this EOT, ultracold ICl molecules below 10 μK with large volume would be obtained.
The hyperfine levels of the J = 0 and 1 levels of the vibronic ground state of I 35,37 Cl are precisely determined by diagonalizing the effective Hamiltonian matrix at the basis of | JI 1 F 1 I 2 F 〉, where the interactions due to nuclear magnetic dipole and nuclear electric quadruple are both taken into account. Subsequently, the Stark sub-levels due to the interaction of the nuclear electric quadruple with the external field are studied by employing the Hamiltonian matrix at the basis of | JI 1 F 1 I 2 FM F 〉 both at the fixed applied electric field of 1000 V/cm and at the applied field varying from 0 to 1000 V/cm. The results would be useful for electrical manipulation (trapping and further cooling) of ICl molecules at hyperfine level.
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 | |
| 18 | |
| 19 | |
| 20 | |
| 21 | |
| 22 | |
| 23 |