†Corresponding author. E-mail: smhu@ustc.edu.cn
*Project supported by the National Natural Science Foundation of China (Grant Nos. 91436209, 21225314, and 91221304) and Chinese Academy of Sciences (Grant No. XDB01020000).
The Boltzmann constant kB is a fundamental physical constant in thermodynamics. The present CODATA recommended value of kB is 1.3806488(13) × 10–23 J/K (relative uncertainty 0.91 ppm), which is mainly determined by acoustic methods. Doppler broadening thermometry (DBT) is an optical method which determines kB T by measuring the Doppler width of an atomic or molecular transition. The methodology and problems in DBT are reviewed, and DBT measurement using the sensitive cavity ring-down spectroscopy (CRDS) is proposed. Preliminary measurements indicate that CRDS-based DBT measurement can potentially reach an accuracy at the 1 ppm level.
The present definition of kelvin is that the thermodynamic temperature of the triple point of water (TPW) is exactly 273.16 K.[1] Because it is very difficult to control the macroscopic quality of the water cells, including the isotopic abundances and contents of impurities, [2] the inconsistency among different national primary TPW cells can be as large as 0.1 mK. Mills et al. proposed[3] to redefine the kelvin unit on an exact value of Boltzmann constant kB, which directly relates the thermodynamic temperature to thermal energy. In a similar way, base units of kilogram, ampere and mole will be redefined by linking them to exactly known values of the Planck constant h, elementary charge e, and Avogadro constant NA, respectively. The new definitions would be independent of any material substance, techniques of implementation and environments. The proposal has been accepted by the International Committee for Weights and Measures (Comité international des poids et mesures, CIPM). As a rule, new definitions should be based on the values of the fundamental constants agreeing with the best available measurements, to maintain the units invariant to current definitions.
The kB value under present definition of kelvin is 1.3806488 (13) × 10– 23 J/K recommended by CODATA2010.[4] It has been determined using different methods. Acoustic gas thermometry (AGT)[5] determines the speed of sound in a gas at thermal equilibrium, by measuring acoustic resonant frequencies of a cavity, usually cylindrical or spherical, in which the acoustic resonator eigenvalues are known from theory. The experimental approach of AGT is to determine the speed of sound c0(T, p) of a monatomic gas, usually helium or argon, at around the TPW temperature and at different pressures, and an extrapolation to the zero pressure limit gives c0(T, p = 0). The molar gas constant R = kBNA can be derived according to the equation
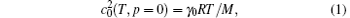
where M is the molar mass of the gas, γ 0 ≡ Cp/Cv is the ratio of the specific heat capacity at constant pressure to that at constant volume, which is 5/3 for ideal monatomic gases. Since the relative uncertainty in NA is only 4.4 × 10– 8 in CODATA2010, kB can be derived from the R value determined in AGT. Dielectric constant gas thermometry (DCGT)[6] is similar to the refractive index gas thermometry (RIGT).[7] They measured the polarizability of 4He, and kB is derived from the comparison to theory.[8] DCGT technique measures the change in capacitance of a capacitor with and without helium gas, and RIGT measures the index of refraction of helium gas in a microwave resonator. Johnson noise thermometry (JNT)[9] is a purely electronic approach, which determines the quotient of the Boltzmann and Planck constants, kB/h, in which h has a relative uncertainty of 4.4 × 10– 8 in CODATA2010. JNT measures the Johnson noise voltage in a bandwidth of frequency across a resistor in thermal equilibrium at TPW temperature.
The present CODATA2010 recommended value of kB is inferred from a group of results obtained from AGT, [10– 15] RIGT, [7] and JNT.[9] The combined relative uncertainty of kB is 0.91 ppm. An uncertainty of 0.71 ppm based on AGT method has been recently reported.[16] The CODATA2010 included kB values and recent results[6, 16– 19] are presented in Fig. 1.
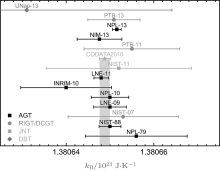 | Fig. 1. The Boltzmann constant kB determined by different groups using different methods. INRIM: Istituto Nazionale di Ricerca Metrologica (Italy); [14] LNE: Laboratoire national de mé trologie et d’ essais (France); [12, 15] NPL: National Physical Laboratory (UK); [10, 13, 16] NIM: National Institute of Metrology of China; [18] NIST: National Institute of Standard and Technology (US); [7, 11] PTB: Physikalisch-Technische Bundesanstalt (Germany); [6, 17] U-Nap: Seconda Università di Napoli (Italy).[19] |
Since the uncertainties in the RIGT and JNT values of kB are 9.1 ppm and 12 ppm, respectively, there is an increasing concern that the new value of kB may be solely determined from AGT measurements. As recommended by the Consultative Committee of Thermometry (CCT), the redefinition of the kelvin should be based on measurements applying different types of primary thermometry, to avoid the risk of unrevealed systematic deviation in one single method. Therefore, measurements using alternative methods other than AGT, with sufficiently low uncertainty (< 7 ppm), are required to fulfil the CCT conditions.
An optical determination of the Boltzmann constant was first demonstrated by Daussy et al.[20] in 2007. Denoted as the Doppler broadening thermometer (DBT), it determines the Boltzmann constant kB from the Doppler width of a transition of atoms or molecules in thermodynamic equilibrium at the TPW temperature. The Doppler width, γ D (full width at half maximum, FWHM), relates with kB and the temperature T following the equation:
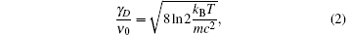
where ν 0 is the central frequency of the transition, m is the mass of the molecule, and c is the speed of light. Note that in Eq. (2), the speed of light, defined as 29979.2458 m/s, is a constant without uncertainty, masses of quite a few atoms and molecules have been determined with an accuracy at the 10– 8 level or better, [4] and frequencies of many atomic or molecular transitions can be straightforwardly measured with an accuracy better than 10– 9, therefore, precise measurements of the sample temperature T and the Doppler width of the transition will result in a spectroscopic determination of kB.
In 2007, the Université Paris 13 group (France) obtained a kB with a relative uncertainty of 2 × 10– 4[20] by measuring an absorption line of NH3 near 10 μ m with a frequency-stabilized CO2 laser. By using a multi-pass cell to improve the signal-to-noise ratio (SNR) in the absorption spectrum, they reduced the uncertainty to 5 × 10– 5.[21, 22] They recently proposed that the accuracy can possibly be improved to 1 ppm.[23] Yamada et al. reported an optical determination of kB with a relative uncertainty of 1200 ppm[24] by measuring a 13C2H2 line near 1.5 μ m with a comb-stabilized diode laser. The Italian group at Seconda Università di Napoli obtained a kB with an accuracy of 160 ppm[24] by measuring an absorption line of CO2 at 2 μ m with a distributed feed-back (DFB) diode laser. Recently they have improved the accuracy to 24 ppm[19] by measuring an absorption lines of 
To the best of our knowledge, all the reported DBT measurements are based on direct absorption spectroscopy. We proposed that cavity ring-down spectroscopy (CRDS) is more advantageous to an optical determination of the Boltzmann constant.[26] CRDS has been first implemented by O’ Keefe and Deacon[27] in 1988. The main idea of CRDS is to measure the decay rate of the laser light emitted from a resonant cavity composed of two high-reflectivity mirrors. The absorption coefficient, α , of the sample gas can be obtained by measuring the ring-down time, following the equation:
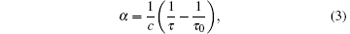
where c is the speed of light, τ and τ 0 is the ring-down time with and without absorption, respectively. The sensitivity of CRDS, often denoted as the noise equivalent absorption, can be as good as 1 × 10– 11 cm– 1· Hz– 1/2.[28] Consequently, for a line with a central absorption coefficient of 10– 6 cm– 1, it is possible to determine the α value with a relative uncertainty at the ppm level.
To record a precise line profile, it is also necessary to achieve sufficient frequency precision during the spectral scan. It can be accomplished by locking the laser to an external reference, either a frequency-stabilized laser[29] or a thermo-stabilized etalon.[30] We have proposed to apply a laser-locked cavity ring-down spectrometer as a Doppler broadening thermometer.[26] The main advantage of using CRDS instead of conventional absorption techniques is that the superior sensitivity of CRDS allows precise measurements with low gas pressures and narrow-linewidth near-infrared lasers. Measurements with low gas pressures will reduce the uncertainty from the complicated collision broadening effect which has not yet been well investigated with a precision at the ppm level. Using mature narrow-linewidth near-infrared lasers secures the frequency accuracy in the measurement which also helps to reduce the statistical uncertainty.
In this section, we will investigate the sources of uncertainties which need to be considered for DBT measurements toward an accuracy at the 1 ppm level.
In DBT measurement, it is crucial to record the line profile with sufficient accuracy. The vertical resolution is related to the signal-to-noise ratio (SNR) achieved in the measurements. In a direct absorption measurement, usually the dominant noise source is the fluctuations in the laser power, which is typically at the level of 0.01%– 0.1%. However, a simulation shows that a SNR of 30000 is necessary for DBT measurements with 10– 6 accuracy.[26] In particular, some residual amplitude variation in the frequency scan cannot be removed simply by averaging, and it may induce a systematic deviation in the recorded line profile. Moretti et al. implemented an intensity control feedback loop to compensate the power variation and they achieved a stability of 10– 4, but the statistical uncertainty (16 ppm) remains to be the leading one in their uncertainty budget.[19] Nonlinear response, either from the detectors or from the amplifiers, also results in distortions in the recorded absorption spectrum. Simulations indicate that such nonlinearity could induce a systematic deviation in the line-shape and the derived Doppler width.[26]
Cavity ring-down spectroscopy is immune to the power fluctuation of the light source, since it measures the decay rate of a single shot which is irrelevant to the initial light intensity. Furthermore, the resonant cavity significantly enhances the effective absorption path length. CRDS has allowed to measure the absorption spectrum with an unprecedented sensitivity to the level of 10– 11 cm– 1· Hz– 1/2.[28, 31, 32] As a result, spectra with considerably high SNR can be recorded by CRDS even at very low pressures of sample gases. Low-pressure measurements are crucial in DBT measurements to reduce the influence from collisions (will be discussed later). Because CRDS measures the change in decay rate instead of the change in light intensities, the influence due to nonlinearity in detection circuits could also be eliminated.[26]
To achieve an accuracy at the ppm level in the line width, it is essential to acquire a comparative accuracy in frequency, which means to use a narrow-linewidth laser and to calibrate the frequency precisely. Typical width for a near-infrared transition of light molecules like H2O is several hundred MHz, therefore an accuracy of a few kHz is necessary. It means to maintain a frequency stability within a few kHz during a spectral scan with a range of a few GHz to cover the whole profile of the absorption line. Such a frequency accuracy cannot be achieved by a commercial optical instrument, and a practical solution is to covert the optical frequency into the microwave range. Moretti et al. locked a reference laser to a stabilized cavity, and used the beat signal between the probing and reference laser to control the frequency of the probing laser. But a 1-MHz broadening to the laser emission was observed, which induced a 10 ppm uncertainty in their determined kB value.[19] By using a narrow-band laser, locking the laser frequency to a reference, and converting the optical frequency to the microwave region for frequency calibration, spectral scan with GHz-level range and kHz-level accuracy is feasible.[33] Therefore, the contribution to the uncertainty in kB from the frequency precision could be eliminated (< 1 ppm).
The DBT method is based on the assumption that the line width is completely from the Doppler broadening, which only holds true at the zero pressure limit. Since the absorption spectra have to be recorded at certain pressures to achieve sufficient signal-to-noise ratio, collision-induced effects also contribute in the line profiles and result in systematic deviations from the Doppler-induced Gaussian profile.[23, 34– 36] To achieve a DBT determination of kB with competitive accuracy, one needs to determine the Doppler width γ D with a relative accuracy of 10– 6. Even at very low pressures, the pressure-induced broadening needs to be considered. The well-known Voigt profile is a simplified form integrating the Doppler and pressure broadening, but its accuracy is far from satisfactory for DBT measurements. Collisions also induce a narrowing effect (Dicke narrowing), and two simplest models are often applied to take into account this effect: the “ soft” collision model and the “ hard” collision model, which are described by the Galatry profile[37] and Rautian profile, [38] respectively. The real line profile is further complicated by speed-dependent collisions which correlates the Doppler shift and the collision-induced broadening and shifting. Various line-shape models taking into account the speed-dependent collisions have been developed (see Refs. [39]– [41] and references therein). However, it remains a great challenge to validate the realistic line profile from an observed spectrum. Sophisticated theoretical line-shape models can fit the spectrum with a high quality, but it is still questionable about the physical meanings of the fitted parameters.
It is possible to retrieve the γ D value at the zero pressure by extrapolating the Doppler widths derived from fitting of the spectra recorded at low pressures using simplified profiles. Cygan et al. has concluded[35] from a simulation that a good value of γ D even using oversimplified line profiles. But the residual systematic deviation is relevant to the selected molecular transitions and it is still essential to measure at pressures as low as possible. In the most precise DBT measurement to date by Moretti et al., the contribution to the uncertainty budget due to the line profile model has been estimated to be about 15 ppm (type B).[19] Since a sample pressure of a few hundred Pa was applied in that study, we can expect that the uncertainty due to line profiles could become less significant at much lower pressures. DBT measurements based on cavity ring-down spectroscopy can reduce the necessary sample pressures by at least two orders of magnitude, owing to its greatly enhanced sensitivity. But the systematic instrumental deviations of the CRDS apparatus need to be further examined for profile measurements toward the ppm level.[40]
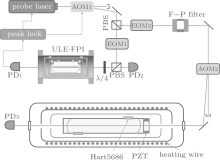
In a DBT measurement, the gas sample should be kept at the TPW temperature. The uncertainty in temperature directly contributes to the uncertainty in determined kB. The sources include temperature fluctuations during the measurement, uncertainty in the calibration of the thermometers, and temperature gradients along the gas sample cell. The temperature of the sample cell should be measured at an accuracy of 1 ppm or higher. The thermometer should be reliable and precisely calibrated at the TPW temperature. We have built a heat-shielded gas cell of over 50-cm long using standard platinum resistance thermisters (Hart 5686) and a readout (MKT 50) from AntonParr Inc for temperature measurements. After calibration, the uncertainty of the recorded temperature has been reduced to 0.5 mK and can be improved to 0.1 mK in the future.
It is also important to select a proper atomic or molecular line for DBT measurement. First, the natural width of the line should be negligible, otherwise it must be considered in the modelling of the line shape. The line also needs to be an intrinsically single line. Any hyperfine structure or Zeeman shift due to stray magnetic field could induce considerable deviations in the line profile. In this respect, a ro-vibrational transition of a closed-shell molecule is more appropriate for DBT measurements. The transition should also be truly isolated. Note that even a very weak line in vicinity of the main target line will cause a significant systematic distortion, which will make the derived line width larger than it should be. The parasitic weak lines can be due to molecules as contamination in the sample, minor isotopologues, or weak lines in a hot band. Due to the limited dynamic range, there could be numerous “ hidden” weak lines in the shadow of a relatively strong line given in a spectroscopic database like HITRAN, [42] particularly for a polyatomic molecule. It is essential to carry out a careful investigation of the nearby weak lines around the selected main strong line.
We are developing a CRDS instrument for Doppler broadening thermometry and the configuration is presented in Fig. 2. The ring-down cavity is composed of a pair of mirrors with a reflectivity of 0.99995. The 50-cm long cavity is installed in a vacuum chamber with a three-layer structure to maintain a temperature stability of better than 1 mK. From outside to inside, the first layer is a stainless steel vacuum chamber and it is also used as a heat sink. The second layer is made of aluminum and it is heated with a wire attached on its surface. A feed-back circuits is applied to stabilize the temperature of this aluminum layer by controlling the current in the heating wire. The third layer is also made of aluminum, and it is used as a heat shield to isolate the RD cavity from the residual temperature drift of the environment. Two platinum resistance thermisters are placed at each side of the RD cavity with a separation of about 40 cm. The thermisters and the readout (MKT 50, Anton Parr) have been calibrated with the Chinese national TPW reference in National Institute of Metrology of China. We have tested the temperature stability of our RDC at 300 K. The recorded typical temperature drift on the second layer is within 10 mK, while the temperature fluctuations of the ring-down cavity can be less than 2 mK within a few days.
The probe laser is locked to a longitudinal mode of a Fabry– Pé rot interferometer (FPI) using the Pound– Drever– Hall (PDH) method. The slow and fast feed-back control signals are delivered to the laser controller and the driver of an acousto– optical modulator (AOM 1), respectively. The 10-cm-long FPI is made of ultra-low-expansion glass (ULE) and installed in a high vacuum chamber which is thermo-stabilized at about 303 K with a temperature drift below 5 mK. The frequency drift of the longitudinal modes of the ULE-FPI is estimated to be less than 10 kHz within several months.
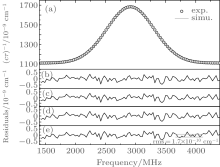
Figure 3 shows an example of the recorded spectrum of acetylene near 12696.4123 cm– 1, which is around the R(9) line of the ν 1 + 3ν 3 band of 12C2H2. The sample pressure is 3 Pa, resulting with a pressure broadening width γ P estimated to be about 1 × 10– 6 cm– 1, which is over 4 orders of magnitude less than the Doppler width γ D. In this case, if a Voigt function is applied for the line profile, and the resulted line width is approximately 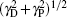
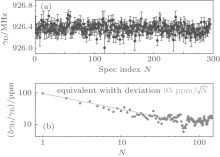
The spectrum shown in Fig. 3 was obtained from a spectral scan during about 100 s. The Gaussian width could be derived from fitting of the observed spectrum with a Gaussian function. Results from about 300 scans obtained in about 10 h are depicted in the upper panel of Fig. 4. A statistics of the results shown in the same figure indicates the relative uncertainty of the derived Doppler width, δ γ D/γ D, roughly follows 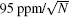
International Committee for Weights and Measures (CIPM) accepted a proposed new definition of the unit kelvin based on a fixed value of Boltzmann constant kB, which relates the thermodynamic temperature to thermal energy. At the present stage, it is necessary to determine the value of kB using different methods to check the consistency, avoiding any possible systematic deviation inherited from a single method. The Doppler broadening thermometry (DBT) determines kBT by measuring the Doppler width of an absorption line of atoms or molecules. The method takes the advantage of rapid progress in precision spectroscopy and laser techniques, and could potentially achieve an accuracy comparable to the acoustic methods. There are miscellaneous sources of the uncertainties in DBT measurements, including the noise and nonlinearity of the detectors, laser line width, and frequency drift, collision-induced broadening, temperature fluctuation and gradients, and weak parasitic absorption lines. The uncertainty in complicated line-shape models due to collisions is the main obstacle for an optical determination of kB with an accuracy at the ppm level.
Cavity ring-down spectroscopy for DBT measurements has some remarkable advantages over the conventional direct absorption method. Its superior sensitivity allows DBT measurements under much lower gas pressures without sacrificing the signal-to-noise ratio, and therefore possibly circumvents the difficulty due to our insufficient knowledge in collision-induced line profiles. We have built a laser-locked CRDS instrument combined with a temperature-stabilized gas cell, which will be applied for DBT determination of kB. Preliminary experiments have been carried out using a ro-vibrational transition of C2H2 near 787 nm using acetylene gas samples with pressures of a few Pa. The results indicate that we can reduce the statistical uncertainty in derived line width to about 10 ppm per day, which is promising for a DBT measurement toward the 1 ppm precision. A cavity ring-down spectrometer working at the triple-point-of-water temperature is under construction in our laboratory.
The selection of proper transitions for DBT measurements is also very important. We are going to use the ro-vibrational transition of carbon monoxide in the 1.56-μ m region, which belongs to the second overtone (V = 3) of CO. These transitions have quite a few advantages that are very useful for DBT measurements: they have moderate line strengths suitable for CRDS measurement; mature narrow-linewidth lasers are available in this spectral region; the inter-molecular interaction of CO is relatively weak, which helps to reduce the influence from collisions; 12C16O has no hyperfine structure and its accurate mass is known; and the relatively simple vibrational band structure of a diatomic molecule eliminates the possible influence from weak lines near the target line. A test measurement shows that we can find some “ truly isolated” transitions of CO in this region: we cannot observe any evidence of weak nearby CO lines with strengths more than one part in million of the target line.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|
| 27 |
|
| 28 |
|
| 29 |
|
| 30 |
|
| 31 |
|
| 32 |
|
| 33 |
|
| 34 |
|
| 35 |
|
| 36 |
|
| 37 |
|
| 38 |
|
| 39 |
|
| 40 |
|
| 41 |
|
| 42 |
|